Analog Devices, Inc. has simplified the design of remote patient monitoring (RPM) devices with the launch of the MAX86178 triple-system vital signs analog front end (AFE) that measures four vital signs with one device. The single-chip AFE integrates three measurement systems (optical, ECG, and bio-impedance) to obtain four common vital signs: electrocardiogram (ECG or EKG), heart rate (ECG or optical photoplethysmography, or PPG), blood-oxygen saturation (SpO2), and respiration rate (using BioZ).
By integrating three clinical-grade subsystems into one IC and enabling synchronized optical PPG and ECG timing, the MAX86178 AFE replaces discrete implementations. The AFE integrates an optical PPG sub-system to measure heart rate and SpO2, a single-lead ECG sub-system, as well as a biopotential and bioimpedance (BioZ) sub-system to measure respiration rate.
In addition, it is housed in a small 2.6 × 2.6-mm package for smaller health monitoring devices. The small solution enables the design of body-worn devices, which can replace medical facility grade monitoring systems to keep people out of the hospital while lowering hospital costs, said Andrew Baker, managing director of the Industrial and Healthcare Business Unit at Maxim Integrated, now part of Analog Devices.
The AFE also enables power savings by providing each sub-system with configurable options to optimize battery life for specific use cases. This allows designers to either use smaller batteries or extend battery life for improved charging.
The device features a very complex set of registers and offers a high level of configurability. “You can configure it for a longer battery life or for higher performance and there’s a lot of configurability in terms of the way the electrodes are configured as well as other performance metrics, Baker said.
By enabling the sub-systems to be configured individually it offers a great deal of flexibility for designers.
Configurability is important because there are usually subtle differences in use cases from customer to customer, including the form factor they’re using – wrist-based versus chest-based versus finger-based, said Baker.
As a 3-in-1 device that is fully synchronous in terms of all the channels, it simplifies the design tasks of designing these multi-vital sign systems, and ultimately the goal is to accelerate the time to market, he added.
The MAX86178 3 AFE builds on the previous generation MAX86176 2-in-1 AFE (incorporated into the Health Sensor Platform 3.0) by adding the Bio-Z channel as well as making improvements to the optical PPG and ECG channels.
“Patient monitoring devices of the past have centered around a few different simple sensing, moving from contextual step counting to heart rate and now we’re getting more advanced sensing in these devices as they become more sophisticated,” Baker said. “It’s about having a more holistic approach to patient health care.”
Use cases
By integrating the three sub-systems in the MAX86178 chip, it opens up new uses cases for remote monitoring and chronic disease management, as the health-care industry strives to deliver better predictive and preventative care, while dealing with rising health-care costs.
There is a big problem in terms of overall costs in delivering health care and a big percentage of that cost is in treating chronic diseases, said Baker. In order to combat and mitigate as well as potentially reduce those costs there is a move away from centralized health care to a decentralized approach, he said.
“The new approach is being pushed forward by the pandemic to try to decentralize [health care] and distribute that care closer to the patient and that brings in a number of things including patient convenience, so they don’t have to travel to these facilities,” he said.
“It also gives an opportunity to clinicians to get a look ahead, so for example if somebody’s predisposed genetically to heart disease or diabetes, you can monitor those individuals and hopefully detect the the onset of these kind of conditions so that they can be managed or medicated to mitigate the risk of those conditions becoming chronic,” he added. “It also gives the clinicians an opportunity to monitor over a longer period of time.”
This has driven the need for new wearables to detect and manage chronic conditions. These devices help clinicians identify the onset of critical conditions to mitigate the risk of hospitalization, which can be costly. One example cited is a body-worn health-care patch that collects vital sign data that is uploaded to the cloud for clinicians to review.
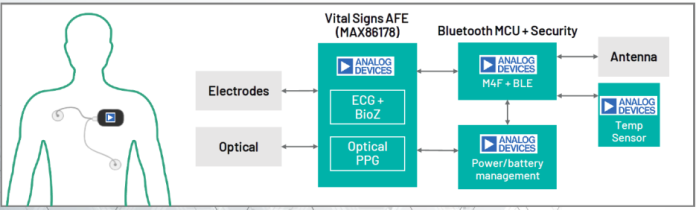
Typical remote monitoring patch system block diagram with the MAX86178. Click for a larger image. (Source: Analog Devices, Inc.)
ADI’s products fall under the umbrella of remote patient monitoring and specifically predictive and preventive monitoring, detecting conditions such as arrhythmias such as atrial fibrillation (AFib), sleep apnea, and chronic obstructive pulmonary disease (COPD).
The key ingredient for these use cases is the measurement of body vital signs, said Baker. The key attributes needed include small size, battery life to fit the use case, and clinical-grade accuracy, he said.

The MAX86178 measures four vital signs: ECG or EKG, heart rate (ECG or optical photoplethysmography, or PPG), blood-oxygen saturation (SpO2), and respiration rate (using BioZ). Click for a larger image. (Source: Analog Devices, Inc.)
The clinical-grade MAX86178 integrates three complete measurement systems – optical, ECG and bioimpedance – and can measure four vital signs simultaneously.
“It is about having a single chip where you can synchronize all three measurement sub-systems [optical, ECG and bioimpedance] with clinical-grade ECG and you can use that ECG for heart rate, but more importantly for cardiac function for things like irregular heartbeats or arrhythmias,” he said.
In addition, the PPG sub-system offers clinical-grade SpO2 oximetry. “SpO2 is becoming increasingly deployed in wearables as the technology is improved. Pulse oximetry is the blood oxygen saturation within the blood, and in order to achieve that you need a very high signal-to-noise ratio, which this particular device offers at 113 dB,” he said.
The bioimpedance channel supports a number of uses cases including impedance cardiography, bioimpedance analysis and spectroscopy, galvanic skin response, and electrodermal activity.
“We believe in this decentralized health care,” said Baker. “The future of wearable health care is starting to accelerate especially with the pandemic, so we need to have more integrated solutions. The outcome that we’re looking for is improved predictive and preventive as well as improved chronic disease management.”
The MAX86178, in a 2.6 × 2.8 mm WLP package, is available now, along with the MAX86178EVKIT# evaluation kit. Analog Devices also offers the MAX20343 buck-boost regulator and the MAX20360 power management IC as power solutions optimized for the MAX86178.
Advertisement
Learn more about Analog DevicesInc.Maxim IntegratedMaxim Integrated Products






