Considerations for selecting and implementing USB standards into medical devices
BY CUAUHTEMOC MEDINA RIMOLDIFreescale SemiconductorGuadalajara, Mexicohttp://www.freescale.com
USB is gaining popularity in the medical market. One of the biggest challenges for designers is compliance with medical industry standards. The Continua Health Alliance (http://www.continuaalliance.org) seeks to unite smart technology and medical devices with healthcare industry leaders to set industry standards for medical systems.
Selecting a standard
When addressing USB connectivity, two important standards are IEEE 11073 and the personal health care device class (PHDC). IEEE 11073 provides structure to the communication interface by defining commands to access data, structuring data to be transmitted and defining communication states. The PHDC has been defined by the USB organization as a standard implementation of USB communication for medical devices in the industry.
Designing specialized USB
The advantage of designing medical applications with a USB stack made specifically for medical USB devices is that it eases medical applications data exchange due to a specific device specialization layer. Designing medical applications under a conventional USB stack might not provide the added value of medical organizations certifications.
Three main factors must be considered when selecting a particular USB connectivity software for medical devices:
1. Standardization . The solution should be based on well-known standards in the industry. This will ensure success and proper introduction from the product to the market.
2. Connectivity . Implementation of the USB should allow multiple devices from different vendors within an ecosystem topology to connect. A connectivity-friendly environment is sustained by a robust, easy-to-use software stack.
3. Portability . Having a multidevice independent layered architecture eases the porting of code between devices. Selecting a hardware vendor with a broad portfolio is key to ensuring customization and product roadmap establishment.
Software architecture ensures code robustness portability and reliability in embedded systems development. In Fig. 1 , several layers of software abstraction isolate the reference application from the low-level communication drivers, permitting code reuse and portability between devices.
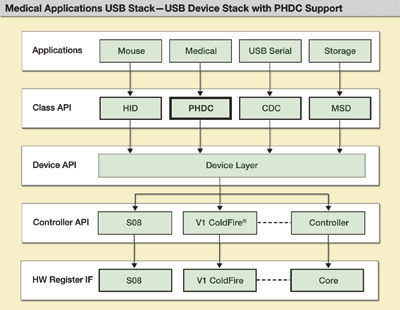
Fig. 1. Proposed software architecture for medical USB class.
An API (application programming interface) consists of the functions that can be used at the device and class levels, which enables implementing new classes. This architecture has four generic class categories: mass-storage device (MSD), communication device class (CDC), human interface device (HID), and PHDC. The API functions defined for these classes can be used to create applications.
The PHDC block in Fig. 1 shows the core of the proposed medical USB stack in terms of medical application operability. The software block provides the basics for medical applications to exchange data. The application usually sits atop the stack. Based on the implementation, the application interfaces with the class driver, the low-level driver, or sometimes the controller directly.
Sample applications
In the future, more USB devices will comply with standards such as IEEE 11073. A sample application featuring a weight scale device has been created to demonstrate the value of working under the standardization scheme and allowing multivendor device interoperability.
The personal healthcare application interacts with the host computer using the IEEE 1107320601 and IEEE 1107310415 weight scale) protocols. It is important to note that the host computer runs the same IEEE 11073 protocols. After this software is installed, the host is emulated and ready to connect the weigh scale device.
Other personal healthcare applications can also interact with the host system. For example, the IEEE 11073 – 10407 (blood pressure monitor), the IEEE 11073–10417 (glucose meter), and the IEEE 11073–10408 (thermometer) protocol.
This interaction lets the medical device vendors gain an advantage while certifying their products with recognized industry standards. ■
Advertisement
Learn more about Freescale Semiconductor





