In what’s being touted as a major breakthrough for solar energy technology, researchers from HZB and TU Delft (Germany) have successfully stored 5% (approximately) of solar energy chemically in the form of hydrogen.

The group was able to achieve this feat using a solar cell and photo anode made up of metal oxide, a system much simpler than the triple-junction cells or III-V semiconductors being used in many of today’s solar-based technologies.
How it works
To accomplish this technology, the team started with a simple silicon-based thin-film cell and added a layer of the metal oxide bismuth vanadate. The only part of the cell that comes in contact with water, its job is to act as a photo anode for oxygen formation.
The system was then coated with an inexpensive cobalt phosphate catalyst.
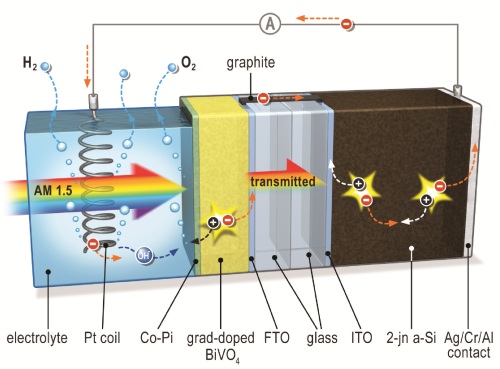
When light hits the system, electrical potential builds. The metal oxide layer is connected to the solar cell via a conducting bridge made of graphite. Since only the metal oxide layer is in contact with the electrolyte (water), the silicon solar cell remains safe from corrosion. A platinum spiral serves as the cathode were hydrogen is formed.
Using sunlight to split water into hydrogen and oxygen is referred to as “artificial photosynthesis.” It allows for the chemical storing of solar energy in the form of hydrogen which, in turn, can then be used directly as a fuel or in the form of methane. It can also be used to generate electricity in a fuel cell.
The team believes that with some fine-tuning, this system is capable of achieving a 9% rate of efficiency.
“Basically, we combined the best of both worlds,” explains Prof. Dr. Roel van de Krol, head of the HZB Institute for Solar Fuels: “We start with a chemically stable, low-cost metal oxide, add a really good but simple silicon-based thin-film solar cell, and — voilà — we’ve just created a cost-effective, highly stable, and highly efficient solar fuel device.”
How well does it work?
Stability of this technology was an issue with this system during development. Specifically, the problem was in how efficiently electrical charges were being separated. Metal oxides, while stable and cheap, yield charge carriers that quickly recombine. When recombination happens, the metal oxides are no longer available for water splitting reaction.
To overcome this hurdle, van de Krol and team added wolfram atoms to the bismuth vanadate film.
“What’s important is that we distribute these wolfram atoms in a very specific way so that they can set up an internal electric field, which helps to prevent recombination,” explains van de Krol.
Once the atoms were applied, the bismuth vanadium wolfram solution was sprayed onto a heated glass substrate. While doing this caused the solution to evaporate, when done repeatedly, and in different levels of concentration, a perfectly usable and highly efficient photo-active metal oxide film some 300 nanometers thick was created.
”We don’t really understand quite yet why bismuth vanadate works so much better than other metal oxides,” says van de Krol. “We found that more than 80 percent of the incident photons contribute to the current, an unexpectedly high value that sets a new record for metal oxides.”
As far as how much energy can be stored, at a solar performance in Germany of approximately 600 Watts per square meter, it was estimated that 100 square meters of this system would be capable of storing 3 kilowatt hours of energy in the form of hydrogen.
Total amount of sunshine needed? One hour.
Looking ahead
The group’s next step will be to scale this system to several square meters so that it can yield usable amounts of hydrogen.
Story via: helmholtz-berlin.de
Advertisement
Learn more about Electronic Products Magazine





