BY JEFF SCHNABEL, CUI, www.cui.com
On August 5, 2013, the U.S. FDA announced the extension of the transition date for IEC 60601-1 3rd edition from June 30, 2013 to December 31, 2013. This means the FDA will accept pre-market submissions with test reports assessed to IEC 60601-1 2nd edition through the end of the year.
The US was set to join the EU and Canada on July 1st in implementing these standards, which better protect the patient and operator, but it looks like some device designers aren't yet ready for this. So, here we examine 60601-1 3rd edition and how will it affect the way you implement power in your device.
What is 60601-1 3rd edition?
The new standard encompasses both hardware and software design of the completed product, and makes some fundamental changes compared to IEC 60601-1 2nd edition:
•”Basic Safety” is now expanded to “Essential Performance.” This is the performance required to avoid unacceptable risk despite the absence of, or degradation of, a function or feature.
•The design process is addressed in more depth, especially for software design.
•The standard is now organized to place greater emphasis on verification and validation of the design.
In short, the revised standard places major emphasis on risk assessment and management. It achieves this by focusing on the development process as much as, or even more than, the final product itself. The Risk Management Process (called out by 60601 and described in ISO 14971) includes a risk management file where identifiable fault conditions are identified and assessed.
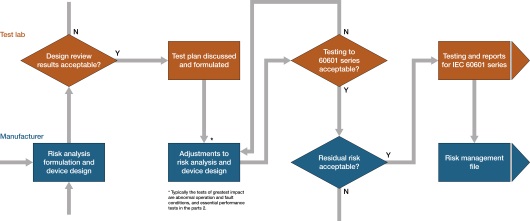
Fig 1: testing procedure flow chart
How does this affect power supplies?
Ac/dc power supplies and dc/dc converters have always played a crucial role in the certification of medical equipment. That's understandable, since the power supply is responsible for major aspects of power conversion, distribution, and protection. Physics 101 teaches us that power (seen both as current and voltage) can be hazardous if not properly managed.
In IEC 60601-1 2nd edition, the guidelines applied when the device was within the “patient vicinity.” Within this envelope, there were three categories of increasing severity: Type “B” (body) equipment operates within the vicinity, but without patient contact; Type “BF” (body floating) devices make physical contact with the patient; and Type “CF” (cardiac floating) equipment make physical contact with the heart. The classification determined what type of levels of isolation, insulation, creepage, clearance, and leakage would be mandated or allowed.
IEC 60601-1 3rd edition changes this perspective by requiring that the overall means of protection (MOP) be some combination of one or more means of operator protection (MOOP) and means of patient protection (MOPP). These can be satisfied with basic safety insulation, use of protective earth ground, and isolation barriers that present a high-impedance path between input and output. Of course, it may be ambiguous if a particular circuit or function falls under MOOP or MOPP categories; the manufacturer needs to assess this and record it in the risk management file.
In practice, the transition from 2nd to 3rd editions does not change the basic requirements on a supply. For Type B applications, a non-medical-rated supply will be satisfactory, as long as it has reduced leakage currents below 500 μA; this is also true for “one MOPP” classifications. Type BF applications will be satisfied by a supply that is rated to IEC 60601-1, which will continue to satisfy “two MOOP” and “one MOPP” classifications. Type CF requires an IEC 60601-1 qualified supply, plus an additional isolation barrier between the supply and the applied part which touches the patient. Typically, this mandate is met with an isolation transformer or a dc-dc converter with 8 mm creepage and double insulation; this is true for the “two MOPP” classification. See Fig. 2 for a summary of these requirements.
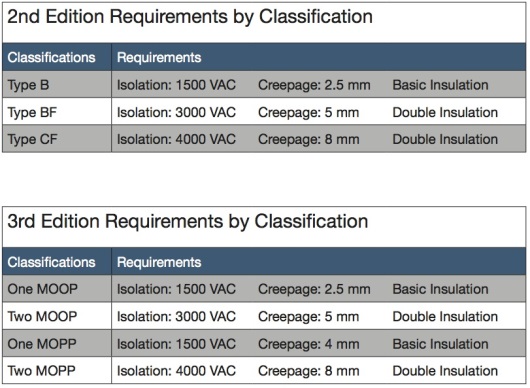
Fig 2: IEC60601-1 3rd edition requirements
As the standard also relates to software it will also have implications for digital power supplies, but the medical market hasn’t really adopted these yet.
Is 3rd edition backwards compatible?
Sadly, no. Some of the requirements of IEC60601-1 2nd edition are in potential conflict with the 3rd. Thus, complying with the latest edition may put a product out of compliance with the 2nd, and so make a product unmarketable in regions that still adhere to the earlier version. Countries still mandating IEC 60601-1 2nd edition include Japan, Australia, New Zealand, and China.
The 3rd edition of IEC 60601-1 has not yet been adopted in China and no clear timetable exists. The Chinese GB 9706.1-2007 standard is however, an endorsement of the 2nd edition IEC 60601-1. It is, therefore, generally not possible to obtain successful registration in China with products developed and documented according to the 3rd edition.
As a result, many vendors are working to meet both versions in the same design, which requires additional time and effort. In order to help ease the compliance process for medical device designers, CUI offers a comprehensive line of medical power supplies ranging from 15~400 W that have been certified to IEC 60601 2nd edition and 3rd edition two MOPP standards.

Fig 3: CUI’s VMS-365 has been designed to comply with both 60601-1 2nd and 3rd edition
For a more in depth application note on the 3rd edition medical standard and how it affects power supply design visit www.cui.com/medical .
Mr. Schnabel also wrote an article about staying on top of the regulations, which you can find at: http://www.eebeat.com/?p=5808
Advertisement
Learn more about CUI Inc





