BY MICAL ANSELMO
Recom Power
www.recom-power.com
The third edition of EN60601-1 safety standard for medical devices and systems has been in force in Europe since the middle of 2012, and has wrought disharmony onto the world – the new standard was not nearly as uniform as it should have been. This article sheds a light on the differences from the previous version, describing how power module manufacturers have addressed the discrepancies.
Standards raise expectations of creating uniform rules and transparency within their scope of application, and in view of this globalized world, it would not have been amiss to expect the same from the third edition of the EN60601-1 standard. Even after years of preparation, there are still astonishing differences around 35 years after IEC/UL 60601 was first published.
Grace for NA a detriment to Europe
The U.S. gave itself an additional year of grace, enforcing the new UL60601-1 on July 1, 2013, while Europe replaced the old second version from 1995 with the third version on July 1, 2012. As if that wasn’t enough, there was another extremely unpleasant difference — the new standard applied to all devices launched onto the European market, even new products with old designs, but the same standard in the U.S. was only applied to newly developed products. The Canadians took the middle road with their CAN/CSA standard by enforcing the standard on the same date as in Europe, but applying it only to newly developed products as in the U.S. Medical devices often linger in product ranges for around five to ten years, so this last point in particular constitutes a costly drawback for European manufacturers — not only because they had to retrofit new components to old designs, but also due to the high additional costs of certifying their new products. This gray zone lasting years is a heavy burden on small companies in particular, which often face the decision of taking current products from the market early while their competitors from the U.S. are allowed to keep on selling their old products.
The situation is even less clear for devices that fall under the EN/UL 60601-2 standard. The third edition is only mandatory for these products once the second edition no longer holds sway for 60601-2 products — which may indeed come later. Some countries haven't or haven't yet enforced the third edition, and their products are still certified according to the second edition. Medical engineering products will literally be subject to double standards for years to come.
Extended risk management regulations
Virtually nothing has changed in technical specifications for insulation; risk management has gained focus as a major area of innovation. Medical device manufacturers will be required to document their risk management process as based on the ISO 14971 model. Specific processes will have to be observed and documented, alongside compliance with fundamental technical standards.
As an example, the second edition allowed devices to break during testing as long as that didn't pose a risk to patient or operator health, but the third edition requires that the system keeps its essential functionality. This has to be documented in a risk management file or RMF, which will also require far more contact between the manufacturer and testing authority throughout the development process — especially as results from the testing process have to be included in the RMF document.
Different standard for patients and operators
Devices with patient contact (means of patient protection, or MOPP) require two isolation barriers for electrical safety, an important requirement in power modules. The first barrier on the supply side has so far been implemented in a medical PSU, while reinforced isolated dc/dc converters provide the second isolation barrier between the medical electronics and diagnostics tools, such as endoscopes and ECG electrodes, to double the safety.
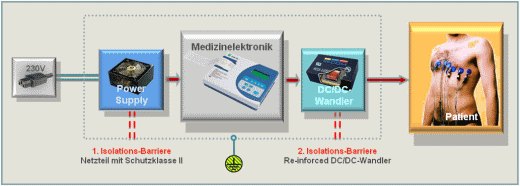
Fig. 1: Block diagram of a medical device for direct patient contact with a reinforced isolated dc/dc converter as the second isolation barrier.
One new feature of the third edition is that it separates off operator protection; in theory, clearance and creepage distances as defined in EN60950-1 for electronic equipment are enough for devices without patient contact intended for protecting operating staff (means of operator protection or MOOP). You might interpret this as a relief, but the requirement for very low operating current also needs to be observed according to EN60601, so manufacturers would be well-advised to limit their possibilities and use medically isolated converter modules in MOOP devices as well.
Reinforced isolated dc/dc converters doubling patient safety
Transformer clearance and creepage distances in reinforced isolated converters have to be three times the size as those used in industrial settings. So far, this has been implemented with primary and secondary windings on opposite sides of a toroidal core separated by a partition wall in the middle. That deals with the isolation issue, but the magnetic fields would not be able to overlap due to the spatial separation between the two windings. An unpleasant side effect would be a decrease in efficiency in the two transformers resulting in increased heat losses.
RECOM developed a small transformer with primary and secondary windings interlocked in such a way as to ensure virtually optimal overlapping magnetic fields despite the creepage and clearance distances required for reinforced isolation. The new converters achieve 15 to 20% more output in the same casing due to quasi-resonant circuit topology, and are also approved for ambient temperatures of up to 85°C — around 15°C more than most transformers with conventional toroidal-core transformers — due to the low heat loss. Depending on type, we were able to reduce isolation capacitance to values down to 1.5 pF, ensuring lower leakage current as required in medical electronics.

Fig. 2: Visual comparison between a reinforced isolated REC3.5 (right) and a single isolation REC3 that is unsuitable for applications with patient contact (left).
A complete generation of highly isolated converters for medical applications has emerged from this new transformer design, such as in programming devices for pacemakers, blood gas analysers, and oximeters. The RxxP and RxxP2 series cover the 1- and 2-W class, and are available in SIP7 casings. The 2-W version is available as an RV series in DIP24 casings for pin compatibility with legacy designs. The REC3.5 and REC6 series both provide 3.5 and 6 W, respectively, and also come in a DIP24 casing. The models quoted are available with isolation voltages up to 8 and 10 kVdc.
Advertisement
Learn more about Recom Power





