For years, engineers have been trying to develop better, cheaper, and more efficient batteries.

Now, Stanford University researchers are saying they are pretty close to doing what all these engineers have been trying to do—develop a pure lithium anode.
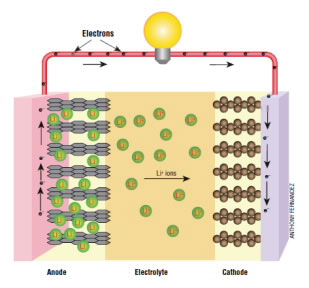
As they explain it, all batteries consist of three parts: an electrolyte to provide electrons, an anode to discharge those electrons, and a cathode to receive them.
Maybe the average battery-user doesn’t know this, but when we say we are using lithium batteries, this is not necessarily true. We are using lithium-ion batteries. The lithium is in the electrolyte, but not in the anode. An anode of pure lithium would be a huge boost to battery efficiency.
 An example of a lithium-ion battery with a graphite anode. (Image via American Chemical Society)
An example of a lithium-ion battery with a graphite anode. (Image via American Chemical Society)
“Of all the materials that one might use in an anode, lithium has the greatest potential. Some call it the Holy Grail. It is very lightweight and it has the highest energy density. You get more power per volume and weight, leading to lighter, smaller batteries with more power,” said Yi Cui, Professor of Material Science and Engineering and leader of the research team at Stanford.
Lithium presents many challenges that make it difficult to use in the anode, but the Stanford team is working around this while others have given up.
The challenges
Lithium-ion batteries have an anode and a cathode separated by an electrolyte. During charging, the positively charged lithium ions in the electrolyte are attracted to the negatively charged anode and the lithium accumulates on the anode. Currently, the anode in a lithium ion battery is actually made of graphite or silicon.
So the team wants to replace graphite or silicon with lithium because the lithium ions expand as they gather on the anode during charging.
The problem is that anodes get fissures during charging and short-circuit the battery, in effect shortening its life.
Also, lithium anodes are highly chemically reactive with the electrolyte so they use up the electrolyte and reduce battery life too.
Not to mention, these kinds of batteries tend to overheat and explode.
What are they doing to work around them?
Stanford researchers built a protective layer of interconnected carbon domes on top of their lithium anode called nanospheres.
The nanosphere layer looks a lot like a honeycomb and has a 20-nanometer-thick wall. (It would take about 5,000 stacked layers to equal the width of single human hair.)
This layer then creates a flexible and non-reactive film that protects it from its challenges.
According to Stanford researchers, it is chemically stable to protect against challenges since it is composed of amorphous carbon which is strong and flexible and can move freely up and down with the lithium as it expands and contracts during the battery's normal charge-discharge cycle.
What does this mean for the consumers?
“In practical terms, if we can improve the capacity of batteries to, say, four times today's that would be exciting. You might be able to have cell phone with double or triple the battery life or an electric car with a range of 300 miles that cost only $25,000—competitive with an internal combustion engine getting 40 mpg,” said Cui.
Stanford’s battery isn’t quite ready for commercial use, but it has made strides in the battery research world.
“With some additional engineering and new electrolytes, we believe we can realize a practical and stable lithium metal anode that could power the next generation of rechargeable batteries,” said Cui.
Advertisement
Learn more about Electronic Products Magazine





