Engineers at Penn State University have developed a fairly efficient method of harvesting low-grade waste heat for power production.

For those unfamiliar, low-grade waste heat is a by-product of many energy generating methods. A good, common example of it can be found in automobiles. When waste heat is generated here in the winter, it gets diverted to run the car’s heating system; however, come summer, this same waste heat must be dissipated to the environment.

On a grander scale, power plants require high heat in order to produce electricity, but once this power gets produced, excess heat needs to be routed to cooling towers in order to be properly dissipated.
The same goes for industrial sites, geothermal sources, and solar generating plants — while the low-grade heat generated might be less than that of a power coal or nuclear power plant, it’s heat — and potential power — that otherwise goes wasted.
“The use of waste heat for power production would allow additional electricity generation without any added consumption of fossil fuels,” said Bruce E. Logan, Evan Pugh Professor and Kappe Professor of Environmental Engineering. “Thermally regenerative batteries are a carbon-neutral way to store and convert waste heat into electricity with potentially lower cost than solid-state devices.”
Specifically, the researchers at Penn State used thermally regenerated ammonia-based batteries that consisted of copper electrodes with ammonia added only to the anolyte (the electrolyte surround the anode).
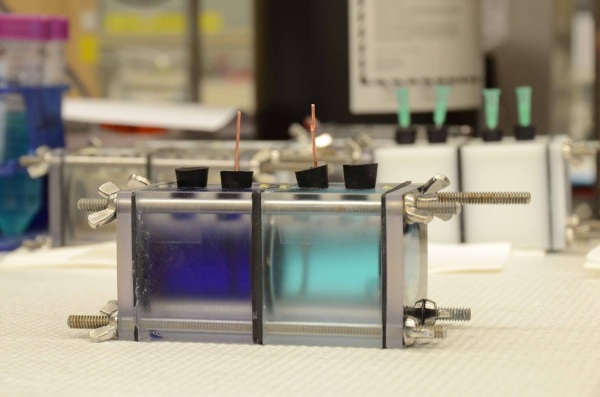
A battery like this is useless as a constant source of electricity if the reaction were not regenerative. Using low-grade waste heat, the Penn State engineers distill ammonia from the effluent left in the battery anolyte and then recharge it into the original cathode chamber of the battery.
The chamber that now has the ammonia becomes the anode chamber and copper is re-deposited on the electrode in the other chamber, now making the cathode (remember, it was formerly the anode). The researchers switch the ammonia back and forth between these two chambers, all the while maintaining the amount of copper on the electrodes.
“Here we present a highly efficient, inexpensive and scalable ammonia-based thermally regenerative battery where electrical current is produced from the formation of copper ammonia complex,” the researchers report in the current issue of Energy and Environmental Science. They note that the ammonia liquid stream can convert the thermal energy to electrical energy in the battery. “When needed, the battery can be discharged so that the stored chemical energy is effectively converted to electrical power.”
“The battery will run until the reaction uses up the ammonia needed for complex formation in the electrolyte near the anode or depletes the copper ions in the electrolyte near the cathode,” said Fang Zhang, postdoctoral fellow in environmental engineering. “Then the reaction stops.”
All told, the thermally regenerative ammonia battery system can convert roughly 29% of the chemical energy in the battery to electricity (so there’s still room for improvement).
In terms of power density, the system was able to generate approximately 60 watts per square meter over multiple cycles; that’s about six times higher than the power density produced by other liquid-based, thermal-electric energy conversion systems.
The researchers noted that the current thermally regenerative ammonia battery is not optimized; they said continued tinkering with it could both produce more power and reduce the cost of the batteries.
Regarding one of the immediate improvements the group was able to make: by increasing the number of batteries, the team was able to increase the system’s total power density. Should this method prove scalable, it just might become something commercial attractive.
Via Penn State
Advertisement
Learn more about Electronic Products Magazine





