 Google’s engineers have produced the first electronic structure calculation performed on a quantum computer, breaking down the energy surface of a molecule of hydrogen using two quantum algorithms. The milestone computation is the first step engineers have taken in modeling reality, having useful applications in various sectors involving chemistry.
Google’s engineers have produced the first electronic structure calculation performed on a quantum computer, breaking down the energy surface of a molecule of hydrogen using two quantum algorithms. The milestone computation is the first step engineers have taken in modeling reality, having useful applications in various sectors involving chemistry.
Scientists have long realized the finite advancement in traditional computing power. For decades, researchers have been working tirelessly to figure out how we can use the enormous potential of quantum mechanics, a fundamental branch of physics concerned with processes involving subatomic particles, to build a whole new generation of computers. Researchers from Harvard, Lawrence Berkeley National Labs, UC Santa Barbara, Tufts, and University College London in the UK working with Google’s engineers used Google’s quantum computer to simulate the energy of hydrogen H2 molecules, a type of prediction often impossible for classical computers or one that would take an extremely long time.
The significance of the feat comes in the expansion of the application; if scientists can repeat the trick for other molecules, the information could benefit research in every field from solar cells to medicine.
“While the energies of molecular hydrogen can be computed classically (albeit inefficiently), as one scales up quantum hardware it becomes possible to simulate even larger chemical systems, including classically intractable ones,” writes Google Quantum Software Engineer Ryan Babbush.
Chemical reactions are quantum in nature, forming highly entangled quantum superposition states. That is, the interactions of particles are so entangled that an individual particle cannot be described independently, and a quantum state must be described for the system as a whole. This is particularly challenging for computers used to dealing in binary values of 1s and 0s. Google’s quantum computer deals in qubits, or bits that themselves can be in a state of superposition, making it possible to represent both 1 and 0 at the same time.
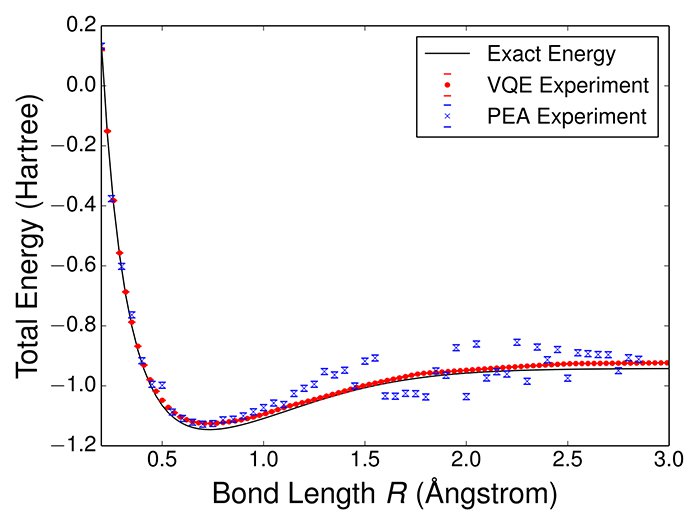
The simulation involved using a variational quantum eigensolver (VQE), a supercooled quantum computing modeling circuit that attempts to mimic the brain’s neural networks on a quantum level. When the results of the VQE were compared against the actual released energy of the hydrogen molecule, computer classically, on a graph, the curves were almost indistinguishable.
 The simulation represents the modernization of the field. Going from qualitative and descriptive chemistry simulations to quantitative and predictive ones is the first step to modeling reality. Google says the applications of this technology could be expansive in a range of fields involving chemistry—from improved batteries and flexible electronics to new types of materials.
The simulation represents the modernization of the field. Going from qualitative and descriptive chemistry simulations to quantitative and predictive ones is the first step to modeling reality. Google says the applications of this technology could be expansive in a range of fields involving chemistry—from improved batteries and flexible electronics to new types of materials.
One potential use discussed in the findings is modeling the way bacteria produce fertilizer. The traditional way human’s process fertilizer is environmentally inefficient, costing 1-2% of the world’s energy per year. If humans were able to understand the chemical reactions involved in fertilizer production, the potential improvements could yield massive gains.
While the computing power of quantum computers is almost unimaginable, some still argue that quantum computers are a figment in the near future as opposed to a physical entity the here and now. Some say the Google’s machine is still a prototype , comprised of part-quantum hardware but can still be categorized as a traditional computer. Both IBM and Canadian company D-Wave have created functioning quantum computers using different approaches. Google’s prototype combines the two main quantum approaches—constructing the computer’s digital circuits using qubits, and an analog approach that encodes a given computational problem into groups of qubits to adjust and shape their collective quantum state to reach a solution, an approach called adiabatic quantum computing (AQC).
Despite the ensuing technical debate, Google’s quantum computer’s ability to accurately simulate chemistry at the quantum level could be one of the most valuable applications of quantum computing.
Sources: Physical Review , Science Alert , Nature
Advertisement
Learn more about Electronic Products Digital





