Of the 21 batteries per year used by the average household, only 5% of rechargeable batteries and 2% of disposable batteries are recycled, resulting in an increasing amount of excess toxic waste without a sustainable mode of elimination. More to the point, the issue will only be compounded by the global battery demand forecasted to rise 7.7% per year until 2019. Now, researchers from Iowa State University have built the world’s first stable transient battery that dissolves in water.
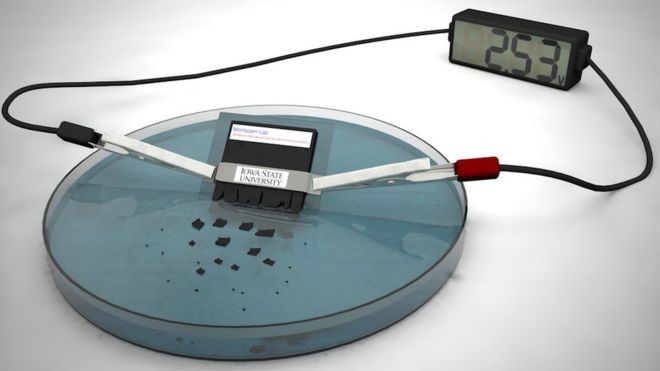
Led by Reza Montazami, an assistant professor of mechanical engineering, the achievement represents a culmination of years of hard work, resulting in a self-destructing, lithium-ion battery capable of delivering 2.5 volts — more than the voltage of an AA or AAA battery — and disintegrating in 30 minutes when dropped in the water. The solution is a dramatic step forward in practicality, doubling the voltage of previous self-destructing prototypes, while systematically reducing the time it takes to dissolve — two steps closer toward a practical solution.
To date, the battery can power a calculator for up to 15 minutes, which, while it may not sound like a long time, still sees a device powered by a dissolvable battery. “Unlike conventional electronics that are designed to last for extensive periods of time, a key and unique attribute of transient electronics is to operate over a typically short and well-defined period, and undergo fast and, ideally, complete self-deconstruction and vanish when transiency is triggered,” Montazami writes in the paper, published in the Journal of Polymer Science .
In its current form, the battery measures 5 mm in length, 1 mm in thickness, and 6 mm in width and is similar to commercial batteries regarding structure and components; it contains an anode, cathode, and an electrolyte separator, uniquely placed within two layers of a water-soluble polyvinyl alcohol-based polymer. Once exposed to water, the polymer casing dissolves, disbursing nano- and micro-particles of lithium salts and silver that make up the electrodes. Incidentally, there’s no mention of any subsequent clean-up process, leaving the question of what to do with the battery residue unanswered. Washing it down the drain or dumping it into the ocean would simply trade one problem for another, which is a rather tough sell.
Therefore, the question that ultimately needs answering is, why might we need self-destructing batteries in the first place? Biotech and military technology present a few of the more obvious applications. Considering that some medical implants are not intended for permanent use, the inclusion of a dissolvable battery may eliminate the need to perform a second invasive surgery to remove the device. A trigger or timer may initiate a countdown to degrade the device over time.
Secondly, military technology armed with dissolvable batteries may function as a kill switch to render the device useless if ever it were seized by an unintended recipient. Assuming the enemy captured a highly computerized weapon system, a single button-press could disarm it, rendering it completely obsolete.
In any case, the technology is too nascent to begin forecasting any tangible application, and the current prototype is more of a proof of concept than anything practical. At the same time, the ability to dissolve even one small battery within a device invites a host of possibilities of not just device control, but possible waste management.
Source: ExtremeTech and BBC
Advertisement
Learn more about Electronic Products Magazine





