The wonder material known as graphene may have a new trick up its sleeve: converting carbon dioxide into liquid fuels. A team of researchers at Rich University in Texas used nitrogen-doped graphene quantum dots (NGQDs) as a catalyst in electrochemical reactions that create ethylene and ethanol, and the stability and efficiency of the material is close to common electrocatalysts such as copper.
In the fight to slow climate change, reducing the amount of carbon dioxide that enters the atmosphere is crucial, and plenty of research is looking into how we can capture carbon at the source, using clay, engineered bacteria, metal-organic frameworks, or materials like the “Memzyme” and sequester it into rock and concrete. Other studies are focusing on converting the captured carbon into liquid hydrocarbons, which can be used as fuel.
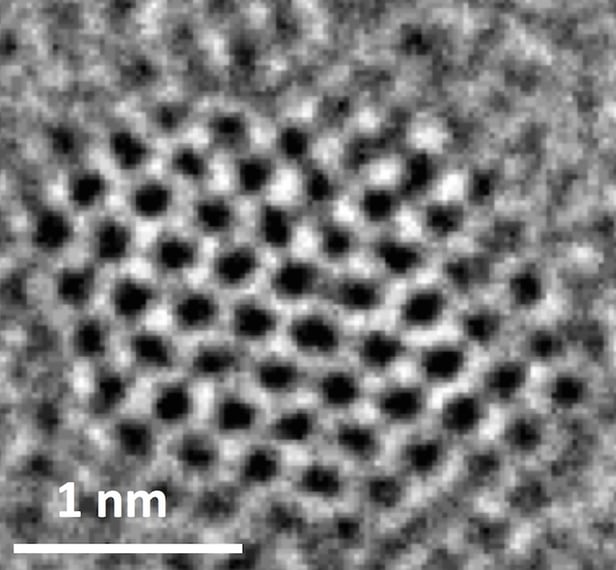
According to the team at Rice University, they’ve found that NGQDs are effective electrocatalysts. The materials are made from sheets of graphene at a single atom thick, divided into dots just a few nanometers wide. Made entirely of carbon, these graphene dots wouldn’t be able to convert carbon dioxide alone, so the team added nitrogen atoms into the mix, which trigger chemical reactions in response to an electric current and carbon dioxide.
“Carbon is typically not a catalyst,” said leader of the study, Pulickel Ajayan. “One of our questions is why this doping is so effective. When nitrogen is inserted into the hexagonal graphitic lattice, there are multiple positions it can take. Each of these positions, depending on where nitrogen sits, should have different catalytic activity,” he said. “So it's been a puzzle, and though people have written a lot of papers in the last five to 10 years on doped and defective carbon being catalytic, the puzzle is not really solved.”
Even though how it all works may remain a mystery for now, the results are promising. Out of the several materials that were tested as electrocatalysts, copper remains one of the main contenders. In terms of efficiency, the NQGDs were found to perform to about the same level as copper, reducing the amount of carbon dioxide released by up to 90% while converting 45% of that captured into small quantities of ethylene and ethanol, which could then be used as fuel. Additionally, the NGQDs were found to keep this up over a long period of time.
The research was originally published in the journal Nature Communications.
Advertisement
Learn more about Electronic Products Magazine





