By Gary Elinoff, contributing writer
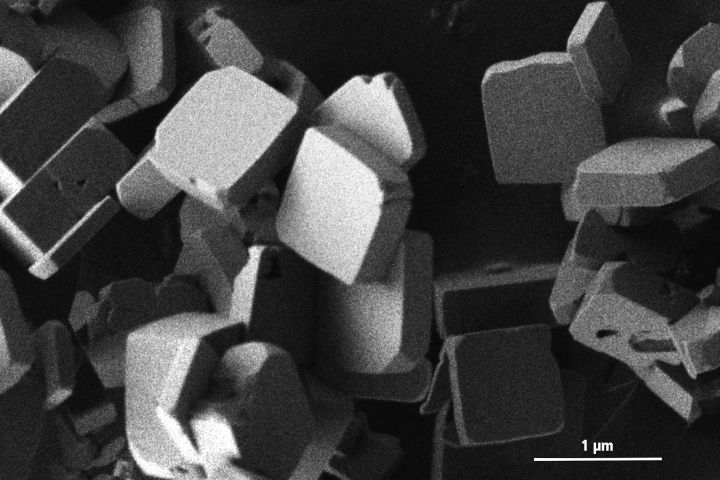
Lithium cobalt phosphate crystals viewed with an electron microscope. Image source: Katia Rodewald/TUM.
It’s no secret that the limited storage capacity of rechargeable batteries is perhaps the single greatest factor impeding the more widespread adaptation of electric vehicles. It’s also the reason why, in the designing and development of chips for any type of mobile device, beyond any other goal is the need for the finished product to require as little operating power as possible.
Lithium cobalt phosphate electrodes
Lithium-ion batteries (LIB) employing cathodes composed of lithium cobalt phosphate have long been an engineering favorite because of their higher energy density when compared to the now-ubiquitous devices employing lithium iron phosphate cathodes, also known as the beltway battery. The comparison is stark; lithium cobalt phosphate batteries can store about 800 watt-hours of power for each kilogram of battery weight, while the lithium iron phosphate design clocks in at about 600 watt-hours.
Until now, producing lithium cobalt phosphate has been difficult. The process needed to take place at 800°C and produced crystals of unpredictable size. Those crystals, in any case, then had to be reduced to a nanocrystalline powder in a subsequent manufacturing process that required a large energy investment.
Synthesizing crystals in a microwave oven
As described in an article from the Technical University of Munich (TUM), Dr. Jennifer Ludwig has found a way to produce nanocrystalline lithium cobalt phosphate crystals more quickly, and the method only requires heating to 250°C, which Dr. Ludwig generated in an ordinary microwave oven. The process also obviated the previous method’s second step as the crystals produced were smaller than 1 μm across, with a thickness measured in hundreds of nanometers. “This shape ensures better electrochemical performance because the lithium ions need to move only short distances within the crystals,” said Ludwig.
But there were some problems along the way. An undesirable compound of cobalt hydroxide hydrogen phosphate was occasionally created by the high pressures involved in the synthesis. Fortunately, Ludwig was able to modify the procedure to avoid its production. “With this new production process, we can now create high-performance, platelet-shaped lithium cobalt phosphate crystals with tailored properties in high quality,” said Tom Nilges, within whose group Dr. Ludwig works.
Another advantage of lithium cobalt phosphate batteries is that they produce somewhat higher voltages than do other LIBs, which is one of the reasons for the increased energy density. Research in LIBs is one of the hottest topics in technology today. Increased safety and speed of charging are also vital concerns, and major automobile companies such as Toyota are taking notice. One thing’s for sure: Power on the go is in demand.
Advertisement
Learn more about Electronic Products Magazine





