Use of MEMS flow meters in a heart replacement system lets stable patients stay comfortably at home, rather than in hospital
BY KAREN LIGHTMAN
Managing Director
MEMS Industry Group
www.memsindustrygroup.org
and
DONNA SANDFOX
Product Manager, MEMS Sensors
Omron
www.components.omron.com
MEMS technology is enabling new biomedical applications that improve quality of life (QoL) in a variety of ways. Providing intelligent sensing and actuation — which can be combined with electronics processing “muscle”–like ASICs, microprocessors, and even DSPs — MEMS enables a high degree of interactivity with the environment. MEMS packs this intelligence into a small footprint, making it the ideal companion for resource-constrained applications.
At a recent symposia convened by the MEMS Industry Group, some of the top innovators in biomedicine explored the use of MEMS in life-enhancing and life-saving QoL applications. Dr. Marvin J. Slepian, co-founder, chairman, and chief scientific and medical officer at SynCardia Systems (www.syncardia.com), delivered a presentation on recent advances in SynCardia’s Total Artificial Heart, a temporary, bridge-to-transplant heart replacement. The advance serves as an outstanding example of the state of the art of MEMS applications in medicine.
A totally artificial heart
As its name implies, SynCardia’s Total Artificial Heart is a total heart replacement for patients who have end-stage heart failure of both ventricles (biventricular failure). The bridge-to-transplant device provides immediate, safe blood flow of up to 9.5 liters per minute (LPM) through both ventricles for patients while they await the availability of a donor heart.
The design of the Total Artificial Heart enables the patient’s body to automatically adjust blood flow according to his or her activity level, providing more blood flow during exercise than at rest. With this improvement in circulation, the health of the patient’s other vital organs often significantly improves, resulting in an overall stronger body and a better transplant candidate.
Both the right and left heart ventricles and the four native heart valves are removed. Then the device is implanted and connected to a driver system outside the body which powers the Total Artificial Heart and contains all of the pumps, motors, and associated electronics.
Miniaturization for patient freedom
The Total Artificial Heart began sustaining patients in the hospital with its 418-lb Big Blue driver in the early 1980s. The Companion, a newer and smaller hospital driver, received the European CE Mark in 2009. This driver is small enough to be pulled on a portable caddy by European Total Artificial Heart patients. The Companion Driver incorporates two 50-LPM MEMS flow sensors from Omron, which are well suited for the application because of their high accuracy, sensitivity and output stability. Omron provided customization of these models to meet the faster response time required for the application.
Further miniaturization of the design continued, resulting in the new 13.5-lb Freedom portable driver (see Fig. 1 ). While heart-failure patients were once hospitalized while awaiting a heart transplant, the new Freedom portable driver allows stable patients to live at home while awaiting their new heart.
CE-approved in Europe, the Freedom driver is currently in an FDA-approved clinical study in the U.S. in which stable Total Artificial Heart patients who meet study criteria may have the option to be discharged from the hospital with the Freedom portable driver, to wait for their matching donor heart at home and in their communities.
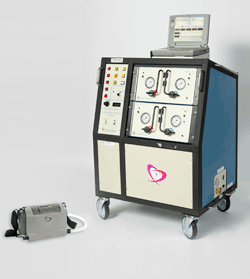
Fig. 1: Small enough to be carried in a backpack, SynCardia’s 13.5-lb Freedom driver (lower left) replaces its 418-lb Big Blue predecessor, thus allowing patients to leave the hospital and remain at home while waiting for a compatible donor heart to become available. [CAUTION: – The Freedom driver is a an investigational device, limited by United States law to investigational use.]
The driver was designed to provide patients with more “freedom” in their daily lives, both inside the hospital and at home. In addition to improving patient comfort, Freedom helps keep healthcare costs down; when stable patients recover in their homes rather than in a hospital, it virtually eliminates in-hospital costs for this portion of patient care. What’s more, it frees up hospital beds to care for other seriously ill patients.
The MEMS factor
The Freedom driver uses the D6F-03A3-000 3 LPM MEMS flow sensor from Omron. Its ultra-compact 36.6 (L) x 8 (W) x 16.8 (H)-mm size, and 5.27-g weight have contributed greatly to making the Freedom driver small and light enough to be carried by the patient in a backpack. The fast-response sensor (under 5 ms) did not require any modifications for the application, allowing the company to use a standard off-the-shelf product (see Fig. 2 ).
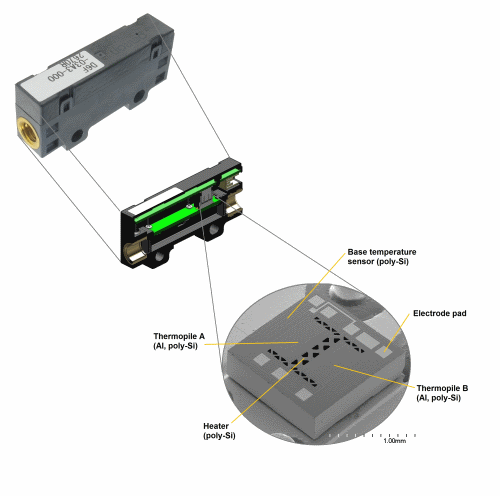
Fig. 2: An off-the-shelf device, the D6F-03A3-000 3 LPM MEMS flow sensor from Omron is ultra-light and ultra–compact, using MEMS thermopiles to detect flow.
The Freedom portable driver has provided mobility to more than 65 Total Artificial Heart patients worldwide, accounting for more than 20 patient years of support. To date, the longest a patient has been supported with the Total Artificial Heart is 1,374 days; Italian patient Pietro Zorzetto received a matching donor heart on Sept. 9, 2011 after living for nearly 4 years with the Total. .There have been more than 950 implants of the Total Artificial Heart, accounting for more than 230 patient years of life.
Thus the Total Artificial Heart is already a significant medical advancement that dramatically improves the lives of patients awaiting donor heart transplants. Moreover, SynCardia’s Dr. Slepian foresees further advancements in the Total Artificial Heart—such as smarter stents with MEMS sensors that would monitor potential membrane failures. ■
Advertisement
Learn more about Omron Electronic Components





