By Gary Elinoff, contributing writer
The most vexing problem faced by users of rechargeable batteries is how long they take to charge. It’s an inconvenience for users of mobile devices, and more so than any other factor, it’s the reason for the slow acceptance of electrically powered cars. A team at Drexel University, headed by Professor Yury Gogotsi, has developed a novel new battery design based on two-dimensional transition metal carbides (MXenes) whose “third dimension” is only a few atoms thick. Batteries with electrodes built with MXene offer the possibility of being able to absorb a full charge in seconds rather than in hours. The paper detailing the work was originally published in the journal Nature Energy.
Electrode architecture based on MXene offers two very important distinctions.
First, as a battery is being charged, electrically charged ions pour into it, looking for a place to call home. MXene makes that journey faster and easier due to its property of macroporosity, or many openings through which an electrically charged ion can more quickly work its way to its “port” on the electrode. That is where it’s stored for later use, when the battery will be providing electricity rather than receiving it.
According to Maria Lukatskaya, first author of the Nature Energy paper, “The ideal electrode architecture would be something like ions moving to the ports via multi-lane, high-speed ‘highways’ instead of taking single-lane roads. Our macroporous electrode design achieves this goal, which allows for rapid charging — on the order of a few seconds or less.”
The second distinction is that with MXene, many more of these ports, or redox sites, can be contained within a given area. The result is that a battery based on MXene electrodes can store more charge for a given size and weight.
As described on Inverse.com, most contemporary batteries use chemical energy storage. MXene, in the form illustrated below, can be described as “microscopic Swiss cheese,” with many storage sites for ions. Then, according to the Inverse article, the application of hydrogel causes the creation of still more of these redox sites, increasing the storage capacity of the battery.
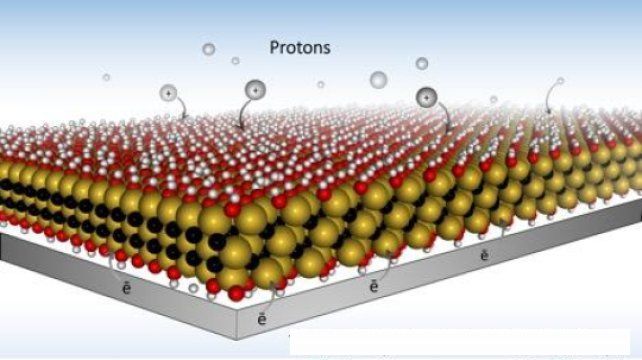
The atomic structure of MXene. Source: Drexel University (edited).
MXene makes it possible to combine the fast-charging and -discharging characteristics of supercapacitors with the superior storage capacity of batteries. As Professor Gogotsi describes it, “This paper refutes the widely accepted dogma that chemical charge storage, used in batteries and pseudocapacitors, is always much slower than physical storage used in electrical double-layer capacitors, also known as supercapacitors.”
As quoted in the Inverse article, “Elon Musk said it best: Existing battery technology sucks.” There have been many false starts in battery technology, and many new ideas turn out to be unscalable, never making it out of the laboratory. Battery technology seems to be the bottleneck on the road to progress in so many fields. So we all wait — and hope — that this innovation may clear the way.
Advertisement
Learn more about Electronic Products Magazine





