
The man credited with developing a biodegradable silicon chip in 2012 has just unveiled a fully biodegradable battery that he and his team built over last two years. John Rogers is a Professor of material sciences and engineering at the University of Illinois at Urbana-Champaign and somewhat of a biodegradable-electronics rock star, having pioneered lots of progress in an area relatively stagnant of late. The batteries seek to mend the inabilities of the silicon chips by surpassing their inabilities.
Rogers’ initial biodegradable silicon chips of 2012 could monitor temperature or mechanical strain, heat up tissue to prevent infection, and radio the results to external devices; however, they relied on induction coils to wirelessly draw power from an external source, thus limiting the devices from being sent deep within tissue or under bone. Similarly, expanding the power source would add bulk to the components and take up too much space; to resolve the issue, Rogers’ and his team created a fully biodegradable battery.
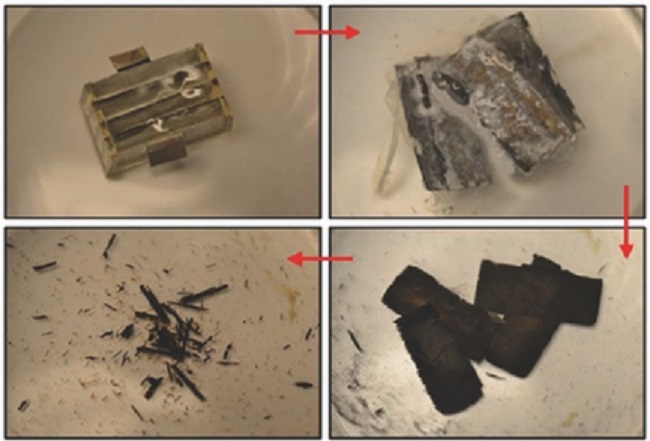
Dissolvable battery
The device is encased in the biodegradable polymer, polyanhydride, and uses anodes of magnesium foil and cathodes of iron, molybdenum or tungsten ─ slowly dissolving metals with biocompatible ions in low concentrations. Finally, phosphate-buffered saline solution is the electrolyte between the two
electrodes.
While the current and voltage depend on the metal used in the cathode, a one-square centimeter cell with a 50-micrometer-thick magnesium anode and 8-micrometer-thick molybdenum cathode produces a steady 2.4 milliamps. Once a battery unit dissolves it releases less than 9 milligrams of magnesium, an amount unlikely to be harmful, says Rogers.
The power generated by the battery remains steady for an entire day, regardless of the metal used in the cathode. “A battery measuring 0.25 cm2 and just one micrometer thick could realistically power a wireless implantable sensor for a day, “quotes Rogers and team in the Advanced Materials journal where device was first revealed. Their ultimate goal is strengthen the batteries’ power density and increase the output by patterning the surface of the magnesium foil to increase its surface area and thus, reactivity.
Applications
A clear-cut application for the battery exists in the form of implanted medical devices that need to dispense medication in response to acute problems, such as epileptic seize, or monitor vital signs. Other uses for the batteries extend to environmental applications; by deploying hundreds of thousands of tiny wireless chemical sensors across oil spills, scientists can track certain characteristics of the spill with the sensors eventually dissolving. A stack of several battery cells can produce 1.6 volts, or enough power to generate a radio signal or power an LED.
Via Nature.com
Advertisement
Learn more about Electronic Products Magazine






