With the recent proliferation of residential solar energy, there is growing interest among general consumers who are looking for ways to store extra energy without having to make a(nother) significant investment.
To overcome this issue, and others similar to it, a group of scientists has put together DIY instructions for creating high-performance batteries made of household items and common scrap metal material.
Their method, a first of its kind, was published in the journal ACS Energy Letters .

The default, go-to choice for storing extra power is lithium-ion batteries. This is because it’s a widely available, widely understood solution for storing energy. The problem in going this route, however, is that it requires a complex, global supply chain, and high-end, sophisticated manufacturing plants.
So, to get around the headache this would involve (using lithium-ion batteries to store solar energy), the scientists decided to go back in battery history to a time when energy storage was a bit simpler in design; specifically, they focused in on the “Baghdad Battery”.
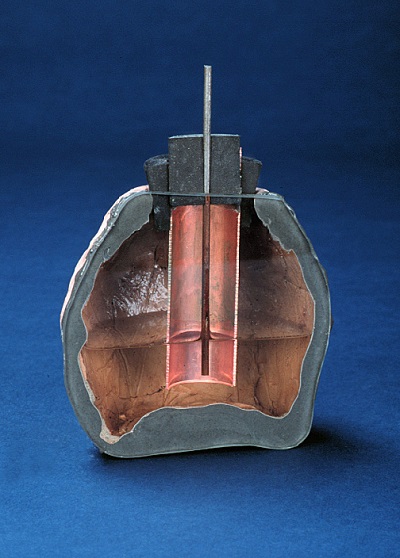
The Baghdad Battery dates back to the first century BC — some believe it to be the world’s oldest battery. It consisted of a ceramic terracotta pot, a copper sheet, and an iron rod, all of which were found along with traces of electrolyte. While the interpretation of these artifacts is controversial, the simple way they were constructed served to influence the research team’s design.
Specific to this particular iteration, the team used scrap steel and brass, the most and third-most abundant kinds of scrap metal waste in the US, respectively.

In the DIY method, the scientists describe a relatively simple process of preparing steel and brass scraps of all shapes and sizes, including shavings and screws, to convert them from waste into effective electrodes for batteries. When combined with aqueous potassium hydroxide — which served as the electrolyte — the result was a voltage of up to 1.8 volts, and an energy density up to 20 watt hours per kilogram. This is approximately the same as that of traditional lead-acid and nickel-iron batteries.
“When our aim was to produce the materials used in batteries from household supplies in a manner so cheaply that large-scale manufacturing facilities don’t make any sense, we had to approach this differently than we normally would in the research lab,” explains Cary Pint, assistant professor of mechanical engineering at Vanderbilt University.
The team is particularly excited about what this method could mean for how batteries are made in the future.
“We’re seeing the start of a movement in contemporary society leading to a ‘maker culture’ where large-scale product development and manufacturing is being decentralized and scaled down to individuals or communities. So far, batteries have remained outside of this culture, but I believe we will see the day when residents will disconnect from the grid and produce their own batteries. That’s the scale where battery technology began, and I think we will return there,” Pint said.
The success of this method can be tied to anodization — a common chemical treatment used to give aluminum a durable and decorative finish. When scrap steel and brass are anodized using a common household chemical and residential electrical current, the team found the metal surfaces restructured into nanometer-sized networks of metal oxide capable of both storing and releasing energy when reacting with a water-based, liquid electrolyte.
In field tests, the scientists’ battery proved it could be charged and discharged more than 5,000 times — the equivalent of over 13 years of daily charging and discharging — and found that it retained more than 90-percent of its capacity.
Given the abundance of the two scrap metals, replacing the DIY battery would be a relatively easy, inexpensive endeavor for any consumer; this, in turn, could help shift the energy storage burden from a centralized model, to a more localized one.
Looking ahead, Pint and team are working towards building a full-scale prototype battery that is suitable for use in energy-efficient smart homes.
“We’re forging new ground with this project, where a positive outcome is not commercialization, but instead a clear set of instructions that can be addressed to the general public. It’s a completely new way of thinking about battery research, and it could bypass the barriers holding back innovation in grid scale energy storage,” he said.
Read the full method, entitled “From the Junkyard to the Power Grid: Ambient Processing of Scrap Metals into Nanostructured Electrodes for Ultrafast Rechargeable Batteries”.
Advertisement
Learn more about Electronic Products Magazine





