Regenerative science reached a new benchmark this month with the unveiling of a 3D-printer capable of transforming simple bone, muscle, and cartilage cells into complex-functioning structures that can thrive when implanted into animals. The device culminates from 10 years’ worth of R&D and solves the most challenging issues surrounding the tissue regeneration; namely, keeping the cells alive in tissue larger than 0.2 millimeters.
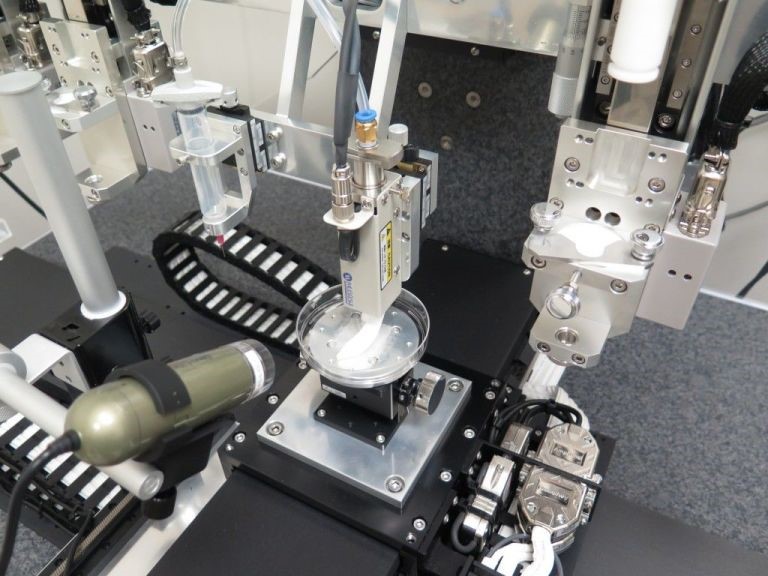
The technology is heralded as “the goose that really does lay golden eggs,” and is thoroughly documented in the journal Nature Biotechnology by Professor Anthony Atala, with his team of biomedical researchers at Wake Forest Institute for Regenerative Medicine. Dubbed the Integrated Tissue and Organ Printing System, or ITOP, the printer uses an additive process similar to other 3D-printers to deposit slowly layer upon layer of tiny droplets containing a rapidly hardening substance.
“This is the first [bio-printer] that can print tissue at the large scales relevant for human implantation,” Atala says. “Once we've printed a structure, we can keep it alive for several weeks before we implant it. Now the next step is to test these [printed tissues] for safety so we can implant them in the future in patients.”
What sets ITOP apart from other bio-printers is the way it structures the layered tissue. First, understand that the supply of nutrients needed to sustain the 3D-printed living cells bottlenecks the size of the fabricated specimen. Previous efforts could construct no more than a 0.2 millimeter mass of cells because contemporary technology cannot print the blood vessels and arteries needed to support larger, more complex structures.
ITOP circumvents these limitations by injecting living stem cells into a gel with sponge-like microscopic valleys that permit blood and nutrients to flow in while a biodegradable plastic framework of polycaprolactone supports the cells as they transform into bone, muscle, or cartilage. The finished product is then kept alive for a few months in preparation for the implantation while the stem cells mature into the desired tissue type. Next, a natural latticework of proteins produced by the cells slowly replaces the plastic. Eventually, blood vessels and nerve cells grow into the implant.
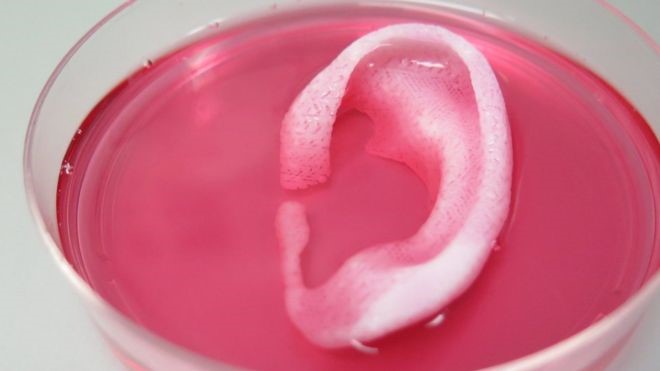
So far, the treatment has yielded success with animal recipients, transforming the stem cells into infant-sized ears of cartilage, chunks of replacement jawbones, strips of muscle tissue, and even bone fragments for the skull. The ear was created from rabbit cartilage embedded beneath the skin of a mouse and left to ripen into its final shape while the mouse’s blood vessels grew into it. Likewise, the same thing occurred when bone tissue printed from human stem cells was implanted within the body of a rat.
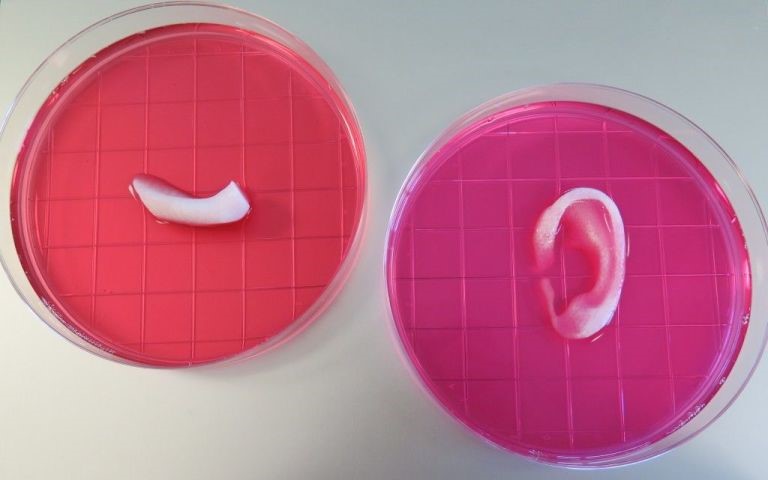
Lastly, the bio-printed strip of muscle—which was the most complex of the samples produced—received blood vessels as well as nerves from the host rat, but did not respond to electric signals; the researchers liken it to new, developing muscles.
Although in the early stages, the results are extremely optimistic for the future prospects of regenerative medicine and are well received by the medical community. Prof Martin Birchall, a surgeon at University College London, told the BBC: “Given the scale of this breakthrough, progress in other fields, the resources available to the researchers at Wake Forest and the imperatives for human health, I think it will be less than a decade before surgeons like me are trialing customized printed organs and tissues.”
As for Atala and the other Wake Forest researchers, the next step is to test the long-term safety of ITOP’s technique to determine the potential for human stem cell implantation. Atala believes that 3D printing customized replacements for bone lies in the very near future.
Source: Popular Mechanics and BBC
Advertisement
Learn more about Electronic Products Magazine





