FDA approves use of TTF device for cancer patients
Alternative cancer treatment that uses electric fields shows tremendous promise
The FDA has approved Novocure’s “Tumor Treating Fields” (TTF) device, which delivers electric fields to slow down — and in some instances, kill — cancerous growths in the brain.
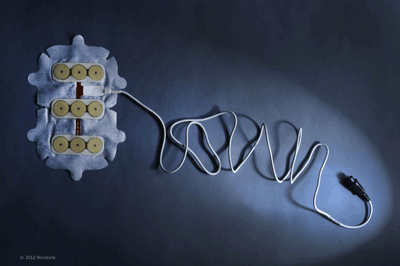
Novocure TTF therapy presents an alternative solution to treating cancerous growths in the brain.
The non-invasive treatment is now available as an alternative procedure for adult patients with recurring brain tumors (recurrent glioblastoma, or GBM).
About GBM
GBM is a particularly aggressive form of brain cancer. It’s also fairly common: The Central Brain Tumor Registry of the United States puts the annual incidence of GBM at 3.11 per 100,000 people, or about 9,500 U.S. citizens per year.
A 237-patient trial indicated that Novocure’s TTF therapy achieved comparable median overall survival times to patients treated with chemotherapy. Also worth noting: there were significantly less side effects, and patients also reported an improved quality of life.
What TTF therapy does
TTF therapy is delivered using non-invasive, insulated transducer arrays, which are placed directly on the skin in the region surrounding the tumor. No electric current is delivered to the tissue, and the device does not stimulate nerves, muscles, or heat tissue.
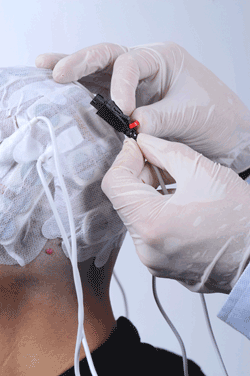
Applying the Novocure TTF device is entirely non-invasive.
Instead, the arrays create an artificial, alternating electric field within the tumor that attracts and repels the charged components of the cells during mitosis (the process by which the cancer cells divide and replicate). The forces that the alternating electric fields exert on the cancer cause a dielectrophoretic dislocation of intracellular macromolecules and organelles during cytokinesis; that is, they alter the cell’s polarity and disrupt its growth. This physical disruption of the cell membrane has proven to lead to cell death, or apoptosis.
Using the device
The Novocure device weighs approximately six pounds. It is entirely portable and designed to be worn by the patient so that it can deliver treatment continuously while the individual goes about their normal, daily activities.


A patient using the Novocure TTF device can easily go about their daily activities.
Patients who’ve undergone TTF therapy report only two side effects when wearing the device: a slight warming sensation at the transducer array site, and a mild-to-moderate rash beneath the electrodes.
How TTF therapy is different from other forms of cancer treatment
1. It’s tuned to affect only one cell type at a time, and has not shown to affect cells not undergoing division in clinical tests.
2. TTF therapy is not expected to affect the normal functions of bone marrow in creating red and white blood cells.
3. It’s delivered locally through a physical, non-chemical pathway.
4. There’s no evidence of cumulative damage to healthy tissues in the body (this can largely be attributed to the fact that since the fields alternate so quickly, they end up having no effect on normal, dormant cells).
Novocure background
Novocure was founded in 2000 by Dr. Yoram Palti, whose concept that a cell’s physical properties can serve as targets for anti-cancer therapy is the basis of the company’s groundbreaking technology.
Today, Novocure is a global, commercial-stage oncology company dedicated to the advancement of TTF therapy for cancer patients.
Outlook
Novocure is preparing an IDE submission for a pivotal (phase III) trial of TTF therapy given in combination with chemotherapy for patients with non-small cell lung cancer.
You can follow the progress of the company’s efforts and learn more about the technology at their website: novocure.com
■
Information and images via the Novocure website.
Advertisement
Learn more about Electronic Products Magazine





