By Patrick Mannion, Contributing Editor
The impetus and opportunity for innovation in mobile healthcare has never been higher, and technology providers are stepping up. New ICs, modules, kits, and algorithms are helping designers develop the next generation of mobile health solutions that will take health monitoring from the doctor’s office to the home, though there are some intriguing issues yet to be resolved.
The many studies on the topic of mobile healthcare (mHealth) show that the market is growing fast. Markets and Markets estimates that between 2015 and 2020, it will grow at a CAGR of 33.4% to $59.15 billion on the back of smart gadgets, mHealth apps used in the management of chronic diseases, the need to manage rising healthcare costs, the penetration of 3G/4G cellular connections, and more interest in patient-centric healthcare.
The study also points to some interesting potholes on the path to mHealth. These include the rising risk of data theft, stringent regulations by the FDA and EU healthcare regulatory bodies, low guidance from physicians in selecting apps, and resistance from traditional healthcare providers.
Despite the potholes, mHealth devices and applications are moving from inaccurate novelties for fitness fanatics to reasonably accurate must-haves for a concerned and aging population. With serious health concerns, it is this population that is much more likely to take a doctor’s advice and keep wearing monitoring devices.
The devices now available for mobile, connected health monitoring include pulse oximeters, blood pressure monitors, peak flow meters, blood glucose meters, sleep apnea monitors, and motion tracking, such as accelerometers to detect a fall or GPS to track Alzheimer’s patients.
The applications are many and varied, but the design issues are familiar: low-cost, low-power, higher-accuracy, and some assurance of security. There’s also an interesting problem that has yet to be resolved effectively: how to guarantee that a urine or blood sample that has been given remotely is truly from the right patient.
Centero, a company that has a history of being a provider of wireless technologies and services for the IoT, is wrestling with this issue right now, even as it deploys CenLab. CenLab, as the name suggests, is a portable, connected healthcare platform comprising the CenLab Fluid Chemistry Analyzer, a CenLab Mobile Application, and CenCloud, a cloud-based Health Information System (HIS) (Fig. 1 ).
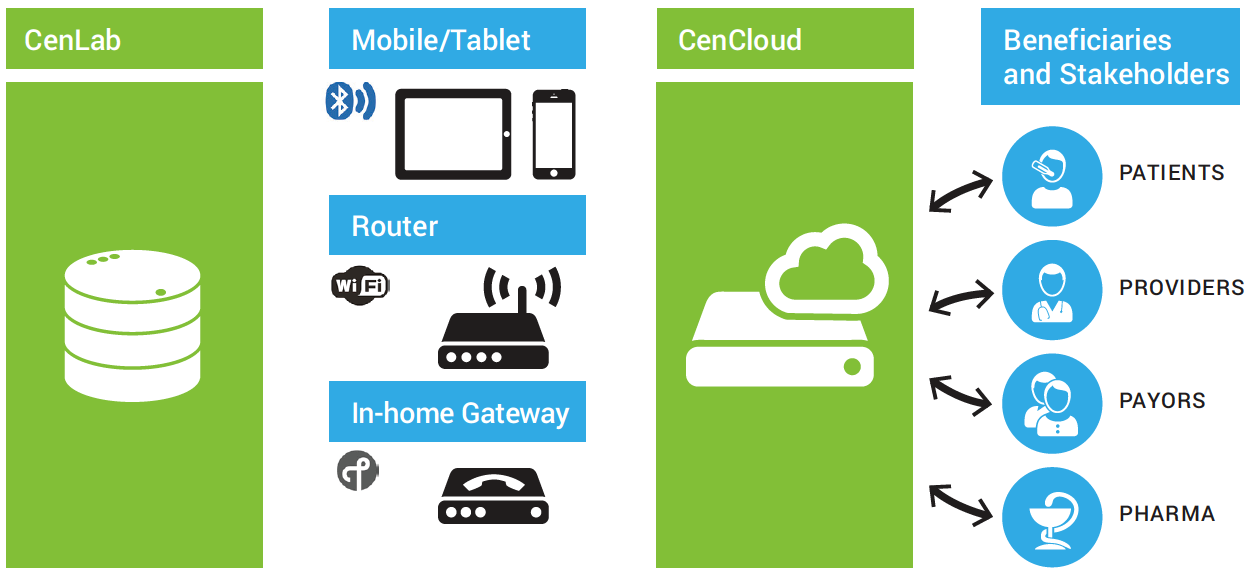
Fig. 1: Centero’s handheld, wirelessly connected fluid chemistry analyzer provides instant urine-based screening to the ordering physician, but there’s a catch. Image source: Centero.
Intended as a versatile tool for pre-emptive screening, CenLab is a fluid chemistry analyzer that enables remote urine-based screening without the patient having to go a lab or a doctor’s office. According to Robert Assimiti, co-founder and chief technology officer at Centero, CenLab helps track the urinary tract, kidney functions, sodium intake, glucose, and a host of other areas of concern.
As useful as CenLab is, while also being on the cutting edge of what mHealth could be, there are a few procedural technicalities and one big catch that need to be resolved.
The procedural issues have to do with FDA regulations. Any device used for diagnostics must go through rigorous and onerous FDA qualification. “Connected health devices are completely different from fitness devices,” said Assimiti. “There’s a wealth of data that must be generated to meet FDA guidelines.” This has always been the case for medical systems, of course, but it continues to be a counter to the burgeoning need for desire for low-cost devices and rapid innovation from a more diverse base of individuals and companies.
Still, companies like Centero are making progress, though an interesting catch that requires some more thinking fits the “elephant-in-the-room” analogy. “One aspect we’re trying to solve is identity: how to ensure a sample is from the subject,” said Assimiti. This is tricky for two reasons: There are natural human and system errors in tracking that must be continuously mitigated. Then there are deliberate attempts by patients to avoid detection of certain issues or chemicals by using samples from someone else. That has yet to be worked out, said Assimiti.
In the meantime, designers interested in developing the next generation of mHealth monitoring systems are being enabled through a range of sensor ICs, modules, and algorithms.
User-friendly heart monitoring
One of the ongoing debates is the relative accuracy of electrocardiogram (ECG) versus photoplethysmogram (PPG) for heart monitoring. An ECG measures the electrical signal controlling the expansion and contraction of the heart’s chambers, while PPG uses reflected light from the skin to indirectly measure heart rate by determining the rate of blood flow. The debate is not about absolute accuracy, as ECG will always be more accurate and is the reference standard, but it is more about the degree to which PPG can be depended upon to give a “good-enough” level of accuracy to allow heart monitoring to move from awkward and uncomfortable chest straps to a wrist-based monitor using PPG.
PPG is already used in hospitals, with a pulse oximeter commonly placed on the patient’s fingertip. Problems arise with PPG when the user is active: Something as simple as an arm swinging can increase blood flow to the wrist and lead to errors. There are other issues, too, such as changing light situations that can affect the photodetector’s ability to separate the LED emissions from ambient light conditions.
That said, these are not new issues, but the techniques and algorithms being developed have greatly improved, led by companies such as Valencell. Valencell specializes in algorithms to compensate for user behavior and usage models, as well as ambient conditions. It estimates that in some cases, the light from the sensor’s emitters may be as little as 1/1000th of the total light collected by the sensor and compares it to finding a needle in a haystack. It uses patented active signal characterization and filtering techniques to find that needle.
Interestingly, it incorporates multiple sensor inputs, such as accelerometers, to arrive at a more accurate estimate, not only of heart rate, but also cardiac efficiency, VO2 , and heart-rate variability.
As Valencell has made progress, the implementation of its algorithms requires highly efficient, powerful processing capabilities and low-power communications. Many semiconductor companies are offering such solutions, with Texas Instruments’ Olivier Monnier, a product-marketing engineer, declaring, “We are recognized as the lowest-power, highest-RF-performance solution on the market.”
Others, like Silicon Labs might take issue with that claim, of course, but with power consumption being so dependent on the application, it behooves developers to perform due diligence, including benchmarking offerings using similar algorithms exercising similar functionality.
This algorithm performance is critical. Colin Tompkins, director of applications engineering at SiLabs, also pointed out the issue of skin color tone, along with ambient conditions, as having an effect on accuracy. Once the processing platform has been selected, the wise approach, he said, is to use statistical analysis across many users and many conditions. “Start with running, and get it working there. Cross-fit is more complicated as its non-periodic noise.”
Silicon Labs has a sensing IC, the Si1143-AAGX, that comprises the optical sensor, associated filtering, analog-to-digital conversion, one 525-nm green LED, and a driver for two additional external LEDs (Fig. 2 ). It also comes with an I2C digital interface and a programmable-event interrupt output.
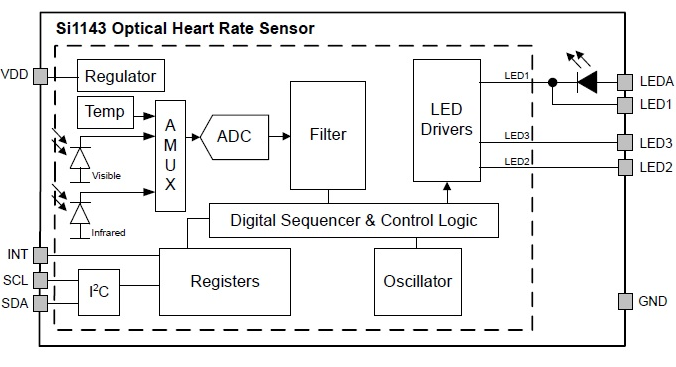
Fig. 2: Silicon Labs’ Si1143 heart-rate monitor integrates the emitter and sensor, along with associated converters and filters, to save footprint but also to better manage optical blocking and minimize ambient lighting effects. Image source: Silicon Labs.
According to Tompkins, having the LED and photodetector onboard solves the problem of integrating optics into a system and of blocking light from inadvertently entering the sensor detector. “You need optical blocking so it [the light] goes through the tissue directly.” The other advantages of on-chip detectors include smaller footprint and less coupling between the AFE and the photodetector. The next generation of modules will have multiple LEDs on board, said Tompkins.
The modulation of the blood flow is under 1%, and while the signal-to-noise ratio (SNR) is a factor of brightness, Tompkins said that the SNR can be dialed all the way down to the 70s (dB) and the system will still get an accurate reading. Under stress conditions (the user working hard), the output power of the LED can be dialed up to get an SNR of 105 to 107 dB. “You can then improve accuracy and do things like HR variability.” Heart-rate variability gives a good view of overall health. Overall, the next-generation module has a >100-dB SNR yet consumes under 100 µA. “And that’s for the entire HR sensing solution,” said Tompkins. “The sensor, AFE photodetector, and LED [including the MCU power to run the algorithm]. That’s state-of-the-art.” A full development kit, based on the company’s Blue Gecko Bluetooth low-energy wireless and Cortex-M4 MCU, is available to help kickstart HRM development using the Si1143.
Note that for darker skin tones, Tompkins said that it might be advisable to use yellow LEDs for better skin penetration, while for earbuds, infrared (IR) light is preferred. Earbud-based HRMs, from the likes of Jabra, Sony and LG, are generally recognized as being more accurate than wrist-based sensors.
Wrist-based ECGs emerging
While PPG is the most popular option for HRM, in large part due to the difficulty of detecting an ECG on the wrist, Xiaomi, working with Huami, has just introduced its Mi Band with ECG-based sensing. Only available in China at the time of this writing, the band costs roughly $100 and comes with IP67 waterproofing and a 95-mAh battery pack.
As expected, power is the most critical aspect of this type of mHealth design, but particularly with ECG sensing at the wrist. Here, the SNR levels required to extract the electrical pulse so far from the heart translate to higher levels of analog and digital processing.
However, companies such as Analog Devices are addressing the needs of ECG AFEs for portable wrist-based designs through devices like the AD8233, a 50-µA, single-lead, ECG front end (Fig. 3 ). While it’s capable of being diagnostic-grade by setting its cut-off frequency to 0.05 Hz, by instead setting the cut-off frequency to 7 Hz, it can be made suitable for fitness applications that detect heart rate only.
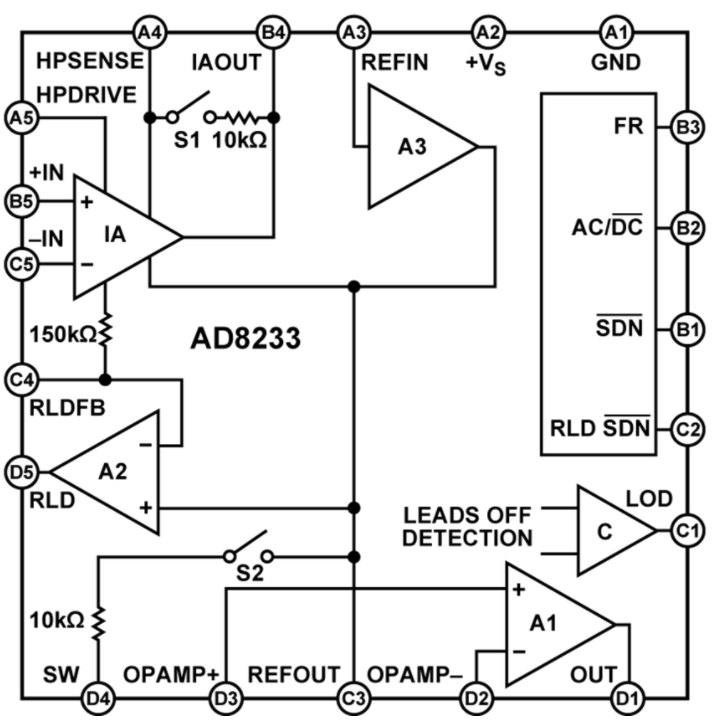
Fig. 3: The AD8233 AFE for ECGs can be set to both diagnostic as well as fitness monitoring by adjusting the cut-off frequency. Image source: Analog Devices.
An included high-pass filter rejects DC half-cell potential that derives from electrode-skin contact. The filter also rejects baseline wander associated with ECG measurements, all while amplifying the ECG signal (1 to 2 mV).
Conclusion
The growth of mHealth coincides with increasing concerns about healthcare in general. As shown, the technology required to address the issues, by making remote monitoring more accessible and accurate, is emerging quickly. Still, designers must navigate the design and regulation waters carefully. That said, technology vendors are quick to provide the necessary guidance.
Advertisement





