By Jon Gabay, contributing writer
Thanks to modern nano-technology fabrication methods and a “scotch-tape” approach to growing it, graphene is becoming readily available as a material for engineers, designers, and pushers of the state-of-the-art to experiment with. This means that we’re at another forefront of technological innovation and an acceleration of capabilities, thanks to this material’s astounding properties.
Graphene is a single layer of carbon atoms bonded together into a hexagonal honeycomb matrix giving it a rather unique set of properties. Although structurally theorized in 1916 and first explored by theoretical physicists in 1947, in the ’60s, it was believed that graphene could not exist because of thermodynamic instability. Then, starting from the ’70s, single layers of graphite were grown epitaxially on other materials, and it was discovered in 2004 that graphene could be extracted from other naturally occurring carbon structures. Since then, amazing properties have been discovered and explored.
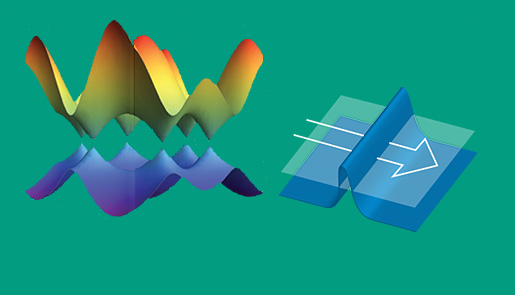
Fig. 1: Electrons pass through graphene as a wave, not as particles, which allows super-low resistance and lets scientists test these “ghost-like” quantum effects — called Klein tunneling — first theorized in 1929.
Touted as the world’s first 2D crystal, the work with graphene earned two researchers the Nobel prize for physics in 2010. Awarded to Geim and Novoselov, they extracted graphene from a piece of graphite. This proved to be the world’s newest form of carbon lattice structures and also the world’s thinnest.
Held tightly together by Van Der Waals forces, graphene is around 200 to 1,000 times stronger than steel (depending on which studies are referenced) for its size and weight in one axis. It’s thought to be the strongest material ever tested. It also exhibits a nonlinear diamagnetism, allowing it to be levitated by strong magnets like neodymium.
In some cases, graphene can act as a pseudo superconductor allowing electronic current flow to transit through with a virtual resistance of zero Ω (10-6 Ω/cm) (see Fig. 1 ). What happens is that electrons passing through graphene act as if they are weightless and can tunnel through the graphene, physically moving at a constant speed of 1,000 km/s. While not as fast as the speed of light, this is much quicker than the rather slow propagation of electrons through a copper wire. And it generates much less heat with higher electrical efficiencies.
In addition to graphene outperforming copper (10x) as a conductor, it also exhibits a very strange effect. Electrical conductivity of graphene can be increased with the application of a magnetic field — a real field-effect resistor. Furthermore, as a tight material, not even the smallest gas atoms can pass through it (bond length of 0.142 nm). At the molecular level, graphene is transparent, absorbing only 2.3% of incident light.
But perhaps its biggest boon for modern electronics are its thermal characteristics. Graphene has been shown to be able to pass 4,840 (±440) through 5,300 (±480) W/m/K. This outperforms silver and copper as a thermal conduit for heat transfer. This is the first feature of graphene to be embraced as designers look at ways to reduce heat with modern electronics.
The more highly dense that a circuit is, the higher its concentration of active devices, and, as these devices get smaller and smaller, the amount of localized heat goes up. Using graphene as a more efficient thermal conduit will allow smaller and hotter circuits to dissipate and radiate heat in a more predictable way over a larger surface area than ever before.
Rutgers researchers have been investigating the use of graphene to cool smart phones, computers, and other electronic chips. They have been able to double the power factor when compared to thermoelectric cooling. They have also verified that graphene is superior for both active and passive cooling techniques.
Future advances will see the use of graphene printers to create on-the-fly intricate and custom patterns. Note that these creations can be for heat, strength, and conductivity purposes. Now if we could make graphene from carbon in the air, we would have a win-win scenario.
Since studying electrical engineering, Jon Gabay has worked with defense, commercial, industrial, consumer, energy, and medical companies as a design engineer, firmware coder, system designer, research scientist, and product developer. As an alternative energy researcher and inventor, he has been involved with automation technology since he founded and ran Dedicated Devices Corp up until 2004. Since then, he has been doing research and development, writing articles, and developing technologies for next-generation engineers and students.
Advertisement
Learn more about Electronic Products Magazine





