Almost a century after metallic hydrogen was introduced as a theory, Harvard scientists succeeded in finally bringing it to reality.
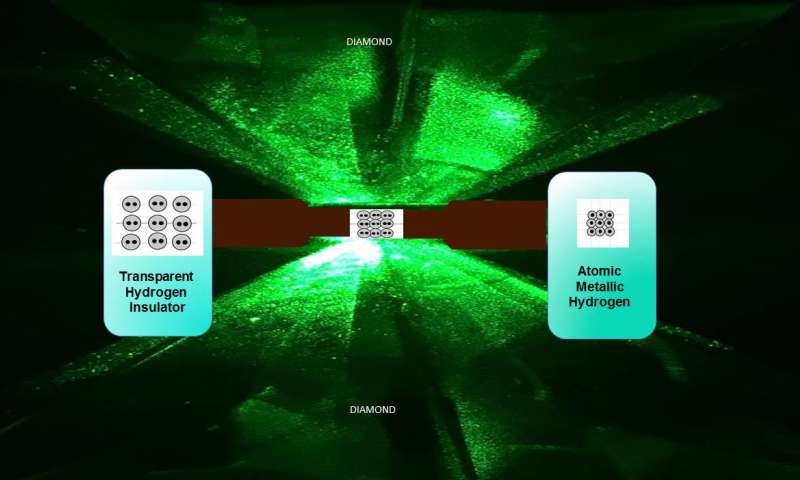
Atomic metallic hydrogen, one of the rarest and most valuable materials on Earth, was created by Thomas D. Cabot Professor of the Natural Sciences, Isaac Silvera, and post-doctoral fellow, Ranga Dias. The material has an extensive range of applications, including being a room-temperature superconductor.
The two created it by squeezing a small hydrogen sample at 495 gigapascals, approximately more than 71.7 million pounds-per-square-inch, a figure greater than the pressure at the center of the Earth. At such extreme pressures, solid molecular hydrogen breaks down and the bound molecules disconnect to transform into atomic hydrogen, also known as metal.
While metallic hydrogen creation allows us to understand the properties of hydrogen better, it also offers hints at potential new materials.
“One prediction that's very important is that metallic hydrogen is predicted to be meta-stable,” Silvera said. “That means that if you take the pressure off, it will stay metallic, similar to the way diamonds form from graphite under intense heat and pressure but remain a diamond when that pressure and heat is removed.”
Predictions suggest that metallic hydrogen could be a superconductor at room temperatures, which would be revolutionary. About 15% of energy is lost to intemperance during transmission, but that could change by making wires from this material and using them in the electrical grid.
Additionally, a room-temperature superconductor has the potential to significantly improve our transportation system, allowing magnetic levitation of high-speed trains, making electric cars more efficient, and improving performance on several electronics.
Because superconductors have zero resistance, energy can be stored by retaining currents in superconducting coils and then be used when necessary. And while it has the potential to transform life on Earth, it could also play a significant role in discovering the most powerful rocket propellant yet.
The most powerful fuels are categorized by a “specific impulse,” the measure (in seconds) of how fast a propellant is fired from the back of a rocket, of 450 seconds. The specific impulse for metallic hydrogen is believed to be 1,700 seconds.
“That would easily allow you to explore the outer planets,” Silvera said. “We would be able to put rockets into orbit with only one stage versus two and could send up larger payloads, so it could be very important.”
To create the new metallic hydrogen material, Silvera and Dias focused on one of the hardest materials on Earth, the diamond. Rather than a natural one, they used two small pieces of polished synthetic diamond that were treated to make them tougher. They were then placed opposite of each other in a device called a diamond anvil cell.
When diamonds are polished with diamond powder, carbon can gouge out from the surface. Silvera noted that when he looked at the diamond using atomic force microscopy, he found defects in it, causing it to weaken and break.
As a solution, they used a reactive ion etching process to shave a minuscule layer (roughly one-tenth of a human hair) from the diamond’s surface. Then they were coated with a thin layer of alumina to keep hydrogen from diffusing into the crystal structure.
Approximately a century after it was theorized and more than four decades of working on metallic hydrogen, the material is finally here.
Source: Phys.org
Advertisement
Learn more about Electronic Products Magazine





