Northwestern University has developed a small, flexible implantable device that relieves pain on demand without the use of drugs. It works by providing targeted cooling to block pain signals to the brain. Measuring only 5 mm at its widest point, the biocompatible device dissolves after it is no longer needed, similar to absorbable sutures.
Researchers said “the first-of-its-kind device could provide a much-needed alternative to opioids and other highly addictive medications.”
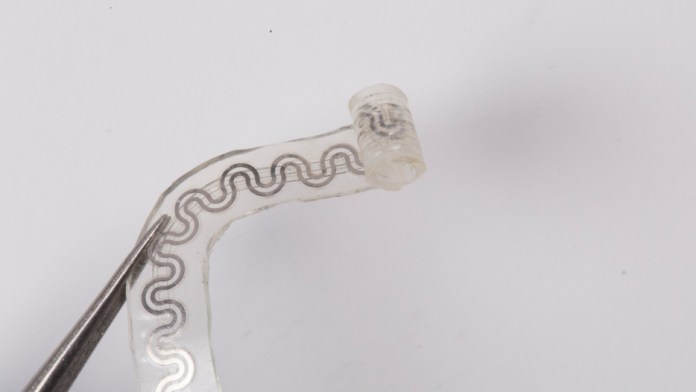
The implantable device is comprised of two separate microfluidic channel: one for the liquid coolant and the other for the gas. When mixed together in a chamber, the liquid evaporates at the specific location of the nerve to deliver precise cooling. (Source: Northwestern University)
The water-soluble device is “softly” wrapped around nerves to deliver precise cooling which numbs nerves and blocks pain signals to the brain, said the researchers. The cooling effect is induced by the two tiny microfluidic channels in the device. One channel contains the liquid coolant (perfluoropentane) and the other channel contains dry nitrogen. When the liquid and gas are combined in a chamber, the reaction causes the liquid to evaporate.
The liquid coolant is clinically approved as an ultrasound contrast agent and for pressurized inhalers, said researchers.
“We are specifically targeting peripheral nerves, which connect your brain and your spinal cord to the rest of your body,” said study co-author Dr. Matthew MacEwan of Washington University School of Medicine in St. Louis, in a statement. “These are the nerves that communicate sensory stimuli, including pain. By delivering a cooling effect to just one or two targeted nerves, we can effectively modulate pain signals in one specific region of the body.”
The device also include an integrated sensor that “monitors the temperature of the nerve to ensure that it’s not getting too cold, which could cause tissue damage,” said researchers.
“Excessive cooling can damage the nerve and the fragile tissues around it,” said Northwestern’s John A. Rogers, who led the device’s development, in a statement. “The duration and temperature of the cooling must therefore be controlled precisely. By monitoring the temperature at the nerve, the flow rates can be adjusted automatically to set a point that blocks pain in a reversible, safe manner.”
Rogers is the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery at the McCormick School of Engineering and Northwestern University Feinberg School of Medicine. He also is the founding director of the Querrey Simpson Institute for Bioelectronics.
An external pump is used by the patient to remotely activate the device as well as to increase or decrease the intensity. When the device is no longer needed, it is naturally absorbed into the body over days or weeks.
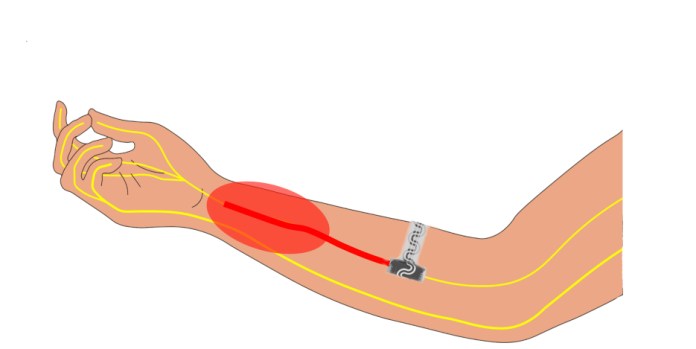
The implantable device wraps around the peripheral nerve to block signals to the brain. The red oval in the illustration indicates the area of pain. (Source: Northwestern University)
Researchers said “the device will be most valuable for patients who undergo routine surgeries or even amputations that commonly require post-operative medications. Surgeons could implant the device during the procedure to help manage the patient’s post-operative pain.”
The research study was published in the July 1 issue of the Science journal. It describes the design and efficacy in an animal model. The study received support from the Phil and Penny Knight Campus for Accelerating Scientific Impact, the Querrey Simpson Institute for Bioelectronics, and the National Science Foundation.
This is the third example of bioresorbable electronic devices from the Rogers lab. The concept of transient electronics was introduced in 2012, followed by a demonstration of a bioresorbable electronic device in 2018 and a transient pacemaker in 2021.
Advertisement





