Lithium- and sodium-based battery technologies edge for market share in the network
BY DAN MCMENAMIN
Dan McMenamin and Associates, Inc.
West Deptford, NJ
The telecommunications network includes the hardwired telecommunications network that’s served us since the 1800’s, our cell phones, smart phones, Blackberries, tablet computers, cloud computing and other emerging broadband wizardry. No matter what part of that network we speak to, some kind of a battery provides standby power for the electronics that convey the voice, video and data streams. For more than a century now, the buildings, roadside huts, underground vaults and cabinets that house the sophisticated electronic systems to process and convey all this information has relied on either lead-acid or nickel-cadmium type battery systems.
Emerging technologies such as lithium- or sodium-based cells and other electrochemistry are forging inroads to the telecommunications markets. Such products promise greater power density, a longer life in perhaps unfriendly thermal environments and ultimately, improved reliability and reduced cost. The purpose of this article is to compare these technologies and let the reader decide whether he or she thinks these newcomers will be the “holy grail” of the network for the next decade or so.
“He ain’t heavy, he’s my battery,” is the mindset of most telephone companies and the lead-acid cell will be with us for a long time to come. The technology is reliable, predictable and relatively inexpensive. In an age of ‘green’ technology lead acid remains a winner because every part of the cell is recyclable and qualified recyclers are easy to find.
What might drive lithium- or sodium-cells is not the cost of lead but the space they occupy and the tendency for lead acid cells to suffer accelerated aging in hot environments. Engineers know well that for each 10°C above 25°C, the battery suffers half-life aging. Thus at 35°C, a twenty year battery will last ten years. At 45°C, that same battery will last five years and so on. Put that battery in an unconditioned cabinet in, say, Yuma Arizona and a useful life of less than a year will be the result. Not so with other technologies. Nickel-cadmium cells tolerate heat quite well. Lithium and sodium cells have internal heaters to provide chemical reactions necessary for their operation. Temperatures we might consider high in a lead acid world are balmy breezes to this technology.
Another factor driving the newer technologies is the burgeoning cost of copper cabling between a communications power source and equipment served. Because lithium and sodium battery technologies don’t mist acid or outgas problematic chemicals, the products open doors to distributing dc power plants collocated with or placed very close to the systems they serve. This capability drastically reduces both the first cost and the lifecycle cost to provide dc power. Collocating dc power with telecommunications central office electronic equipment was tried in the mid-1980’s but failed because the valve regulated lead acid (VRLA) batteries (see Fig. 1) misted electrolyte containing sulfuric acid which, in turn, corroded the equipment.

Fig. 1: Strings of vented lead-acid batteries in a central office installation. (Photo: Dan McMenamin and Associates, Inc.)
Nickel cadmium batteries are an excellent product and enjoy wide applications in the telecommunications network both as a stationary battery and an engine starting battery. The fact that Ni-Cd cells must be watered periodically and, they outgas leaving engineers wary of distributing them close to sensitive electronic systems in central offices, data centers and similar applications.
Lithium batteries are an energy-dense technology that’s gaining interest among battery people. Several white papers were presented on the subject at Battcon 2011, particularly electric utility and telecommunications. The electric vehicle industry is looking to lithium as the Holy Grail because it is packed with power, relatively low in weight and has a very rapid recharge time as compared with heavier, slow to recharge lead acid cells. On the other hand, engineers fret over the possibility that under catastrophic conditions Lithium cells result in dramatic fires. In the electric vehicle realm this threat is essentially risk-neutral when compared with a gasoline tank vulnerable to rear-end collisions.
For telecommunications or electric utility applications, there is no such comparable risk and so those engineers are wary, though encouraged by control system improvements in this emerging technology. Some of that wariness comes from negative experiences seen when a relatively new company in Canada, Avestor, blitzed the industry with a highly touted lithium metal polymer product a few years ago. Initially, the battery showed promise despite a fire in an Atlanta lab. Even the smallest fire events get big press in Telecom. Later, after publishing a white papers at Battcon 2003 and 2004 saying in essence that all the safety and control problems were solved, a couple major players in telecom, initiated field trials and one service provider bought in for a wide deployment. Despite modest sales, the company went broke and those who bought their product scrambled to replace them with more traditional batteries. Industry insiders have expressed opinions that the product was rushed to market, a corporate gamble that doomed them.
The telco and utility markets have been likened to a large ship with a small rudder and it takes time and effort to make that big boat change directions. This fact of industry life is vexing to venture capitalists who live or die by what they call a “Burn Rate,” or the time until money poured into a business begins showing significant returns. Telco and utility have high burn rates.
Having been disappointed a couple of times telcos are putting more effort into vetting new products and especially new technologies entering the network. Time was when both the telcos and the utilities had fairly large engineering organizations and much subject-matter-expert talent to thoroughly research a new offering before integrating it into their networks. Later, as those companies downsized their organizations they experienced a ‘brain-drain’. With fewer specialists to draw upon, companies spread the workload over a smaller base of people. Some called it “increasing one’s span of control,” while others called it having your job “enhanced. Personally, I called the practice “being dilated,” but in any case, people began taking shortcuts.
Often, engineers who gave a new product a cursory examination acceded to vendor pressure and sometimes internal pressure to place a product into a “field trial.” Generally after six months or so if the product hadn’t caused problems they’d authorize a wider use and then soon that product would ‘see’ a full scale deployment. In the case of batteries, sometimes the units in those field trials had never experienced a single discharge because the commercial power grid is reliable. Based upon the experience some had with the Avestor product and others, most users are doing a more thorough job of vetting new products, sometimes outsourcing the task to consultants or labs that they trust.
Lithium-ion batteries are getting some press for stationary battery applications and there are a number of companies offering their version of a product, Sony, Dow Kokam and Ultralife among others. Lithium-ion technology differs markedly from the lithium metal polymer that Avestor chose. With lithium-ion, both the positive and the negative electrodes act as host structures for the Li ions and the Li+ as may be seen in Fig. 2.
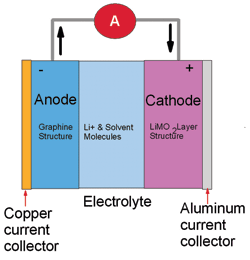
Fig. 2: Lithium-ion technology. (Sketch: Dan McMenamin and Associates, Inc.)
A big concern among stationary battery users is thermal runaway which was a problem in valve regulated batteries but would be disastrous in lithium-ion due to the flammable nature of the electrolyte and the generation of heat between the electrodes. Fires involving lithium batteries are dramatic ones! All cells consist of anodes and cathodes with a separator between them. Ion flow across that separator is what makes the cell an electrochemical cell. In the case of lithium-ion, if too little resistance exists in that path either due to an internal or an external short-circuit, an exothermic chemical reaction will occur and the cell temperature will rise dramatically. As opposed to lead acid or NiCd batteries which use a water-based electrolyte, the electrolyte in a Lithium-Ion cell is flammable. If the electrolyte leaves the cell, exposure to air increases the reaction because the electrolyte can produce a fire. Because the energy density of a Lithium cell is greater than that of its competing technologies, the resulting fire also is much larger in terms of energy .
The electronic protection systems built into Lithium-ion batteries will help to control thermal reactions and thereby reduce the incidence of fire. In addition, in an effort to address flammability concerns, some manufacturers are differentiating their products by encasing cells within battery modules in a thermal management material to inhibit, retard and in some cases potentially prevent the propagation of thermal runaway (Fig. 3). Regardless, quality control issues and operational conditions will rank highly in the conditions that telco engineers vet before agreeing to a wide deployment.
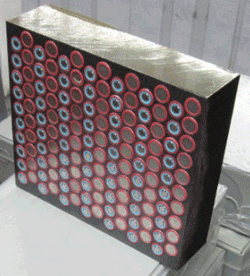
Fig. 3: lithium-ion cells mounted in a thermal management enclosure formed of phase change materials that tend to even-out cell heating. (Photo courtesy: Ultralife Corp./AllCell Technologies.)
As of this moment it appears that lithium-ion will enjoy a bright future in telecom applications such as cell sites or other shelter housed or cabinet type installations. Another potential application is distributed power applications that would drastically reduce copper cabling losses thus reducing first-cost.
Other battery technologies
Sodium nickel chloride (NaNiCl) batteries show great promise for power-dense high temperature applications. Originally, they were developed for the electric vehicle market and Fiamm Corporation is now marketing a product for stationary telecommunications and data center applications. With this technology, the Negative electrode is molten sodium and the electrolyte is a ceramic tube. The Positive electrode is a sodium and aluminum compound and ion transfer through the ceramic electrolyte causes current flow. The technology boasts a power density five times that of lead, a cycle life > 1,000 discharge cycles and a high tolerance for short circuits. Some of the down-sides to this technology is that it has a high internal operating temperature 270°C, necessary to keep the sodium in a molten state. From a cold state, it can take as long as 24 hours to reach operating temperature. The battery consumes as much as 100 W (typically about 55) to keep itself heated. As is the case with lithium-ion, the technology shows promise for distributed architecture systems in central offices where a small dc plant is placed every few aisles eliminating the need for heavy, expensive cabling to a centralized plant.
Another Sodium battery being offered is the Sodium Sulfur cell which is similar in concept to that described above. One downside to this technology is that if the sodium hydride cools too much during a prolonged power loss, the material settles to the bottom of the cell and solidifies there, ruining the cell. While the technology is being watched, at this moment in time Sodium Nickel has more interest from telecom than does Sodium Sulfur.
For both the lithium and sodium based batteries, users will be very watchful to ensure that fire is virtually a non-issue because corrosive smoke is a huge contamination problem. Fires, even as small as a single burning disk drive, can result in the replacement of whole aisles of equipment and more if smoke spreads contaminants.
Engineers must remember that the battery is part of a system and they need to determine their application before deciding on a battery technology. Poor system performance often has its roots in a misapplied battery technology. For example, will the battery see frequent discharge cycles? If so a cycling battery is needed and other technologies will underperform. Will the discharge be a high-rate or a long, slow one? Is the thermal environment outside the battery’s design envelope? These and other system design criteria must be considered if one is building system reliability into their plans. One size doesn’t fit all.
Are electric vehicle and stationary reserve applications the big niches for lithium and sodium batteries? Perhaps right now but they might just lend themselves to creature-comfort camping equipment or electrical ‘build-ins’ for recreational vehicle appliances as the price-point comes down through mass production. The sky is mostly blue for these emerging technologies and telecom, the 600 pound gorilla in the room is testing those skies. Stay tuned! ■
Advertisement
Learn more about Dan McMenamin and Associates





