Researchers at Tsinghua University, China have devised a graphene-based nanostructured lithium metal anode for lithium metal batteries in order to inhibit dendrite growth and improve electrochemistry performance. The findings were originally reported in the journal, Advanced Materials , published earlier this month.
Like Li-S and Li-air batteries, new lithium metal anode batteries are highly sought after, as lithium metal provides a high theoretical specific capacity, which is about 10 times more energy than graphite. However, the issue with this is that the practical applications of lithium metals are hindered by lithium dendrite growth in continuous cycles. This induces safety concerns, since it may cause internal short circuits resulting in fire.
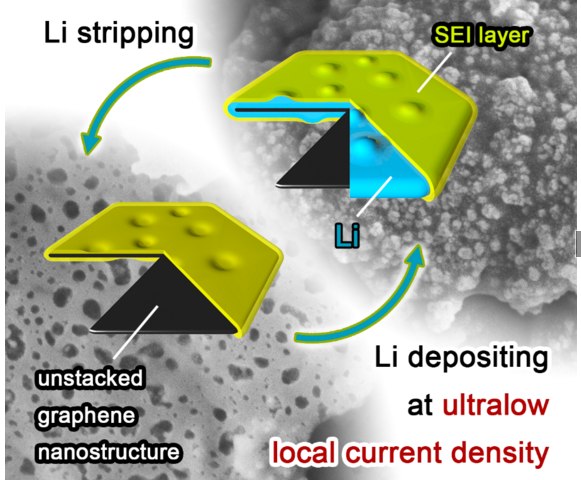
Furthermore, the formation of lithium dendrites induces very low cycling efficiency. This is why inhibiting the dendrites’ growth, as attempted by the researchers in this study, is highly respected.
In the past, many approaches have been proposed to slow the growth of dendrites, including through electrolyte modification, artificial electrolyte interphase layers, and electrode construction.
“We noticed that by decreasing the local current density heavily, lithium dendrite growth could be efficiently inhibited,” said Rui Zhang, a Ph.D. student and author at Tsinghua University. “Based on this concept, we employed unstacked graphene with an ultrahigh specific surface area to build a nanostructured anode. And it turned out to be a very efficient idea. Additionally, we have employed the dual-salt electrolyte to acquire more stable and more flexible solid electrolyte interphase, which can protect the lithium metal from further reactions with electrolyte.”
This graphene-based anode offered great improvement, including (1) ultralow local current density on the surface of graphene anode (a ten-thousandth of that on routine Cu foil-based anodes) induced by the large specific surface area of 1666 m2 g-1 , which inhibited dendrite growth and brought uniform lithium deposition morphology; (2) high stable cycling capacity of 4.0 mAh mg-1 induced by the high pore volume (1.65 cm3 g-1 ) of unstacked graphene, over 10 times of the graphite anode in lithium-ion batteries (0.372 mAh mg-1 ); (3) high electrical conductivity (435 S cm-1 ), leading to low interface impedance, stable charging/discharging performance, and high cycling efficiencies.
The team hopes their research can point out a new strategy to deal with the dendrite challenge in lithium metal anodes. Furthermore, they have also called for additional study of the diffusion of Li ions and electrons in the process of lithium depositing and stripping to advance the commercial applications of lithium metal anodes.
Source: phys.org
Advertisement
Learn more about Electronic Products Magazine





