Low-cost catalyst spurs hydrogen fuel R&D
The discovery is an important development in the worldwide effort to mimic the way plants make fuel from sunlight
Scientists have engineered a cheap, abundant alternative to the expensive platinum catalyst and coupled it with a light-absorbing electrode to make hydrogen fuel from sunlight and water. Reported by theorist Jens Nørskov of the Department of Energy's SLAC National Accelerator Laboratory and Stanford University and a team of colleagues led by Ib Chorkendorff and Søren Dahl at the Technical University of Denmark (DTU), the discovery is an important development in the worldwide effort to mimic the way plants make fuel from sunlight.
Today, most hydrogen is produced from natural gas, which results in large CO2 emissions. An alternative, clean method is to make hydrogen fuel from sunlight and water via photo-electrochemical, or PEC, water splitting. When sun hits the PEC cell, the solar energy is absorbed and used for splitting water molecules into hydrogen and oxygen. The team combined theory and advanced computation with synthesis and testing to accelerate the process of identifying new catalysts. They introduced a “chemical solar cell” designed to capture as much solar energy as possible. The researchers at DTU designed light absorbers that consist of silicon arranged in closely packed pillars, and dotted the pillars with tiny clusters of the molybdenum sulfide. When they exposed the pillars to light, hydrogen gas bubbled up — as quickly as if they'd used costly platinum.
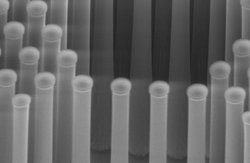
But, the hydrogen gas-generating device is only half of a full photo-electrochemical cell. The other half of the PEC would generate oxygen gas from the water; though hydrogen gas is the goal, without the simultaneous generation of oxygen, the whole PEC cell shuts down. Many groups — including Chorkendorff, Dahl, and Nørskov and their colleagues — are working on finding catalysts and sunlight absorbers to do this well. “This is the most difficult half of the problem, and we are attacking this in the same way as we attacked the hydrogen side,” Dahl said.
Jim Harrison
Advertisement
Learn more about Electronic Products Magazine





