University of California Berkeley chemists have taken a promising new material that captures and stores carbon dioxide and altered it to convert the captured carbon into a chemical useful to the industry. The sponge-like materials called covalent organic frameworks (COFs) can soak up carbon dioxide, making them useful in removing the potent greenhouse gas from power plant emissions or directly from the air. Chemists Christopher Chang and Omar Yaghi, who invented COFs, added a metal catalyst to the crystal structure in order to turn the captured carbon dioxide into carbon monoxide, a primary building block for a wide range of chemical products, including fuels, pharmaceuticals and plastics.
“To date, such porous materials have mainly been used for carbon capture and separation, but in showing they can also be used for carbon dioxide catalysis, our results open up a huge range of potential applications in catalysis and energy,” said Chang, a chemist with Lawrence Berkeley National Laboratory’s Chemical Sciences Division. Yaghi is also a chemist at Berkeley Lab and co-director of the Kavli Energy NanoScience Institute. Chang and Yaghi published their findings in the Aug. 28 issue of the journal Science .
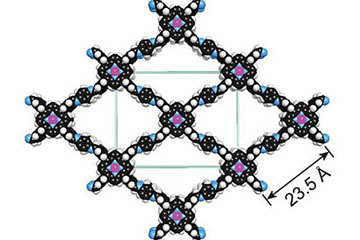
Fig. 1: A covalent organic framework with cobalt atoms added (purple) can both capture carbon and catalyze its conversion to carbon monoxide for industrial processes.
A COF is a porous three-dimensional crystal consisting of a tightly folded, compact framework that has an extraordinarily large internal surface area. A COF the size of a sugar cube, were it to be opened and unfolded, would blanket a football field. The sponge-like quality of a COF’s vast internal surface area enables the system to absorb and store enormous quantities of targeted molecules, such as carbon dioxide. Adding a catalyst, in this case cobalt metal atoms, converted the storage material into an active structure.
A technique called “reticular chemistry,” developed by Yaghi, enables molecular systems to be “stitched” into netlike structures that are held together by strong chemical bonds. Using this, the researchers were able to embed the molecular backbone of COFs with a porphyrin catalyst, a ring-shaped organic molecule with a cobalt atom at its core. Porphyrins are electrical conductors that are especially proficient at transporting electrons to carbon dioxide.
Advertisement
Learn more about Electronic Products Magazine





