By Jon Gabay, contributing writer
The benefits of the miniaturization of electronics and advancements in wireless communications have given us life-preserving and -saving technologies like pacemakers and defibrillators. Technology doesn’t stop here, and more in-body-implanted medical devices are ready to embed in us. But energy needs are still a concern.
That’s why a recently announced development at MIT Brigham and Woman’s Hospital and the Stark Draper Laboratory is so pertinent. They are taking this to the next level by recharging storage cells and powering electronics inside the body using wireless charging techniques.
In this case, they have created a wireless gastrointestinal medical device that can “linger inside the body indefinitely.” It takes readings and can communicate to the outside world using energy derived from the RF field. It is also envisioned that devices like this can store concentrated pharmaceuticals and deliver needed medication either at timed intervals or as an event is detected.
Using an antenna outside the body, researchers were able to transmit commands to an antenna inside the gastric tract. This was able to harvest and condition enough energy to operate a modern low-power micro and sensor array.
According to researchers like Giovanni Traverso, a research affiliate at MIT, “This method yields enough power to run sensors that could monitor heart rate, temperature, or levels of particular nutrients or gasses in the stomach.”
First described in the April 27 issue of the journal Scientific Reports, the paper’s lead author, Abubakar Abid, a former MIT graduate student, says, “Right now, we have no way of measuring things like core body temperature or concentration of micronutrients over an extended period, and with these devices, you could start to do that kind of thing.”
Other approaches examined in charging body implants include using the actual acids inside the digestive system to create a battery, but the obvious issue here is that the corrosive nature of the stomach and digestive tract were too corrosive for long-term feasibility; and inductive charging, the wireless charging technique used with small tablets and phones. Several manufacturers offer the feature for electronic devices, but not for implanted medical devices as the antennas need to be very close (2 to 10 cm apart) for this near-field approach to work. In addition, the efficiency of the energy transfer is directly proportional to the distance separating the antennas.
Source coupling is key, and this team used a technique called midfield transmissions, which takes advantage of power acquisition through a number of electromagnetic inductive and radiated modes. The key is finding an RF band in which the antennas (or coils) don’t need to be too big and the frequency penetrates the body safely with little attenuation. Different types of tissue affect this, too.
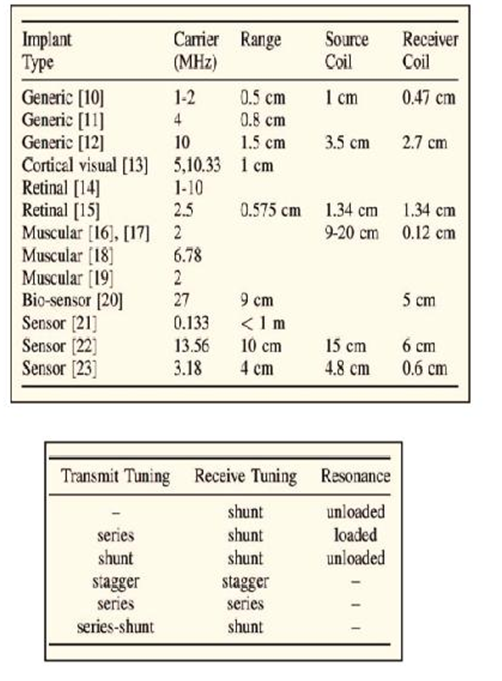
Researchers at Stanford University have researched types of tissue and found carrier ranges for appropriate coil sizes for implantable medical devices for muscular, retinal, and generic uses (see Table 1 ). IEEE senior and student members Ada S.Y Poon, John S. Ho, and Sanghoek Kim were even able to categorize the tuning techniques that would be optimal for this new generation of energy-harvesting medical implants (see Table 2 ).
Tables 1 & 2: Tissue type and carrier frequency can determine how effective a wireless energy transfer system can be to an implanted device inside the body. Tuning modes by receiver and transmitter need to be optimized to maximize energy transfer efficiencies.
More studies and experimentation are underway around the world, and even the use of RF ID Generation II devices may show promise here. The UHF frequencies penetrate the body, and already, monolithic transponder tags can harvest enough energy to transmit back 30 feet away. Non-volatile memory in the transponder is also a plus.
Advertisement
Learn more about Electronic Products Magazine





