For end consumers, a battery is an energy storage unit that provides stored power to an electronic device. Of course, a battery is a finite energy source that will require recharging when depleted. After a period of time or due to frequent use, the battery’s ability to store energy will degrade. When the battery is no longer capable of storing ample energy for the device, it is often replaced. Subsequently, the battery is considered a support structure of the host device and not of primary importance to the overall functionality of the device. 
Fig. 1: Lithium-based battery packs
For battery pack engineers, a battery is a construction that incorporates multiple tiers of protection circuitry to monitor and maintain a miniature electrochemical reactor.
This miniature electrochemical reactor, otherwise known as the cell(s), is the central focus of the battery pack construction. The protection circuitry monitors the operational state of the cells and will internally disconnect the cells from the battery terminals when any safety condition occurs. A cell is manufactured using a particular chemical formula and process. Consequently, each cell has safety thresholds unique to its manufacture.
Typically, the safety circuits monitor for extremes in temperature, cell voltage and electrical current flowing in/out of the cell(s). If the cell is allowed to experience operational conditions that exceed the operational parameters, irreversible damage to the cell(s) will occur and potential gaseous venting, incendiary ignition, or even explosion become real possibilities.
Fortunately, whenever a safety condition is detected by the protection circuitry, the power path from the cell(s) to the battery’s +/- terminals is intentionally interrupted to prevent the condition from escalating to dangerous levels. Most safety conditions are triggered by the transference of electrical power into the cell(s), during charge, or out of the cell(s) during discharge. To allow for eventual recovery from each particular safety condition, the protection circuitry will selectively prevent either charging or discharging. Certain safety conditions (such as overheating of the cell(s) due to environmental heating, or frozen cell(s) due to environmental cooling) will cause both charging and discharging to become disabled until the safety condition is reduced to operational thresholds. Certain safety conditions pose particular concerning hazards, so redundant circuitry is included to protect the Cell(s) in the event of failures in the primary protection circuitry. For example, discharge overcurrent from the cell(s) due to short circuit will require a second level of protection such as a PTC.
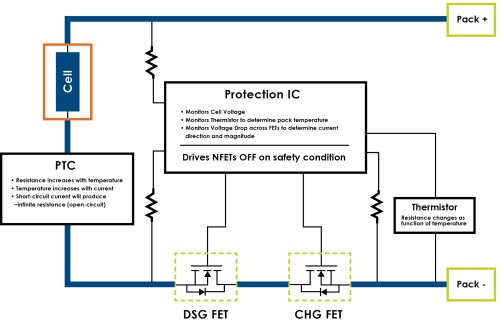
rotection circuitry monitors the operational state of the cells and will internally disconnect the cells from the battery terminals when any safety condition occurs. A cell is manufactured using a particular chemical formula and process and consequently, each cell has safety thresholds unique to its manufacture. Typically, the safety circuits monitor for extremes in temperature, cell voltage and electrical current flowing in/out of the cell(s). If the cell is allowed to experience operational conditions that exceed the operational parameters, irreversible damage to the cell(s) will occur and potential gaseous venting, incendiary ignition, or even explosion become real possibilities. Fortunately, whenever a safety condition is detected by the protection circuitry, the power path from the cell(s) to the battery’s +/- terminals is intentionally interrupted to prevent the condition from escalating to dangerous levels. Most safety conditions are triggered by the transference of electrical power into the cell(s), during charge, or out of the cell(s) during discharge. To allow for eventual recovery from each particular safety condition, the protection circuitry will selectively prevent either charging or discharging. Certain safety conditions (such as overheating of the cell(s) due to environmental heating, or frozen cell(s) due to environmental cooling) will cause both charging and discharging to become disabled until the safety condition is reduced to operational thresholds. Certain safety conditions present hazards of particular concern, so redundant circuitry is included to protect the cell(s) in the event of failures in the primary protection circuitry. For example, the potential danger of extreme over-current discharge from the cell(s) due to an external short-circuit warrants the inclusion of a redundant over-current protection feature, such as a PTC.
Fig. 2: Block diagram of the safety circuitry found in a simple single-cell battery.
Battery technology has enabled portability and power redundancy for a wide range of electrical products. A battery is designed for a particular application and must be thoughtfully evaluated before being re-purposed for an alternative application. Such an evaluation must take into account the safety thresholds designed into the battery, the cell chemistry, and the environmental limitations of the various components within the battery. When utilized within operational parameters, batteries are very safe and allow for otherwise impossible technological concepts.
Advertisement
Learn more about Palladium Energy





