Recently, the U.S. Department of Energy set benchmarks for what storage materials must be able to deliver on in order to be used in post-fossil fuel vehicles.
The list is long and while it was originally thought it’d be a while until consumers were provided a solution that met all the criteria, it appears researchers at Rice University have already come up with a solution.
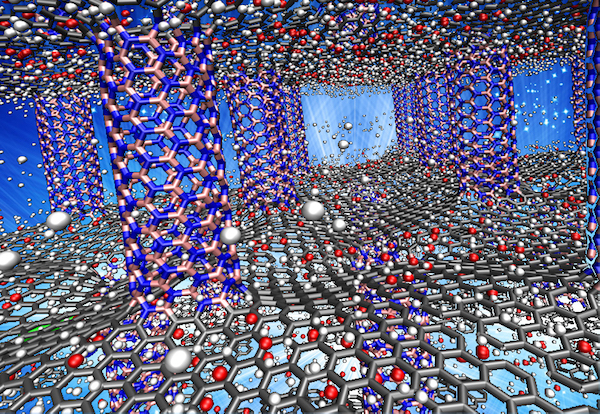
The team has developed a nanomaterial which consists of layers of graphene separated by nanotube pillars of boron nitride. In computer demonstrations, the team used models of 3D architecture of the hybrid nanomaterial to prove it can store enough hydrogen to become a practical fuel for light-duty cars.
Per the Department of Energy, a medium must be able to stare at least 5.5% of its weight in hydrogen to be considered economically feasible. The model the Rice team demonstrated showed it can support nearly 12% of its weight in hydrogen at room temperature; when the temperature is brought down to −196 degrees Celsius, the percentage increases to 15%.
In order to achieve these numbers, the researchers played around with a few different combinations of nanomaterials. The models tested were pillared structures of boron nitride and pillared boron nitride graphene doped with oxygen or lithium.
The best combination proved to be oxygen-doped boron nitride graphene.
During demonstrations with un-doped pillared born nitride graphene, the hydrogen atoms boded to the material due to van der Waals forces; these are forces of attraction or repulsion between molecules unrelated to covalent or ionic bonds. When oxygen is used to dope the material, though, the atoms form a very strong bond with the hybrid material; this results in the creation of a better surface for incoming hydrogen.
“Adding oxygen to the substrate gives us good bonding because of the nature of the charges and their interactions,” said Rouzbeh Shahsavari, a materials scientist at Rice, in a press release. “Oxygen and hydrogen are known to have good chemical affinity.”
Shahsavari adds that the hybrid material allows for a large degree of turnability, making it possible to tailor it for specific operating temperatures and pressures.
The team concluded that they are confident that, based on the material’s structure, it will be able to meet the Department’s requirement that a fuel cell must be able to withstand 1,500 charge-discharge cycles.
To learn more, read the team’s study, “Oxygen- and Lithium-Doped Hybrid Boron-Nitride/Carbon Networks for Hydrogen Storage,” which was published in Langmuir.
Via Rice University
Advertisement
Learn more about Electronic Products Magazine





