By Aalyia Shukat, contributing writer
A group of scientists at the National Renewable Energy Laboratory (NREL) have discovered a technique that allows Magnesium (Mg) batteries to be recharged. By generating an artificial metal-electrolyte interphase to protect the anode’s surface, the team was able to prevent the buildup of a chemical barrier formed during discharge that blocked recharge efforts. The recently published article, “An Artificial Interphase Enables Reversible Magnesium Chemistry in Carbonate Electrolytes,” in the prestigious Nature Chemistry journal notes the successful reversible Mg chemistry of assembled prototype cells.
“This finding will provide a new avenue for magnesium battery design,” said Seoung-Bum Son, first author and scientist at NREL. The other co-authors from NREL include Steve Harvey, Adam Stokes, and Andrew Norman. “The dominant lithium-ion battery technology is approaching the maximum amount of energy that can be stored per volume, she said, so “there is an urgent need to explore new battery chemistries.”
Magnesium-based batteries have several advantages over the prolific lithium-ion (Li-ion) batteries, including cost-effectiveness (Mg is the fifth most abundant element on earth) and a higher volumetric capacity (3,832 mAh/cm3 versus 2,061 mAh/cm3 for Li-ion). However, Mg batteries have traditionally had the major disadvantage of the formation of a surface layer from the metal-to-electrolyte chemical interaction.
Typically, recharging a battery forces the ions at the cathode electrode back to the anode to a point at which there is enough electrochemical potential. Li-ion batteries have a permeable layer, or solid-electrolyte interphase (SEI), between the metal and electrolyte that allows for ions to pass while preventing the electrolyte from being reduced.
For Mg batteries, the decomposition at the interface of the metal generates a blocking passivation layer in Mg batteries that prevents any reversible electrochemical reaction from occurring. Furthermore, in the past, a metal-solid interphase was not feasible because the divalent Mg2+ ions typically could not penetrate the layer. And, in scenarios in which the Mg ions could flow in a reverse direction, it was through a highly corrosive liquid electrolyte, which prevented the successful implementation of a high-voltage Mg-battery.
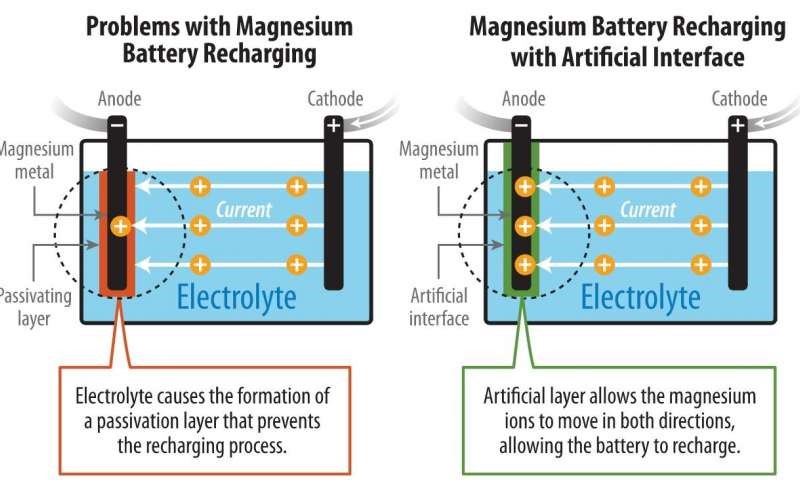
Illustration by John Frenzl/NREL.
The novel interphase made from polyacrylonitrile and magnesium-ion salt demonstrated a highly reversible Mg chemistry in oxidation-resistant electrolytes. The team at NREL successfully deployed the artificial interphase in a Mg/V2 O5 full cell in the water-containing, carbonate-based electrolyte to enable reversible cycling.
Advertisement
Learn more about Electronic Products Digital





