Beamforming in medical ultrasound
BY DANIEL KREINDLER, Samplify Systems,Santa Clara, CA
www.samplify.com
Beamforming is a comon signal processing technique used to create directional or spatial selectivity of signals sent to or received from an array of sensors or antennae. These arrays can be found in many different devices that transmit and receive either electromagnetic or acoustic waves. Thus, beamforming is employed in such varied applications as radio-astronomy, radar, wireless communications, sonar, seismography, and medical and industrial ultrasound. Ultrasound beamforming is unique among these various applications. In order to achieve high-quality ultrasonic images, received beams must be focused dynamically, and the aperture of the array must be amplitude-weighted (apodized) dynamically as well… http://www.nxtbook.com/nxtbooks/hearst/projectmedical_vol3no1/index.php?startid=5
The SMK9130 development kit allows designers to evaluate and use Samplify’s AutoFocus beamformer.Wireless communication challenges in healthcare
Wireless Communication Challenges in Healthcare
BY ANNE HUANG and LEO ESTEVEZ, Texas Instruments, Dallas, TX
www.ti.com
Wireless connectivity is increasingly pervasive in many new markets as mobile phones, consumer electronics, and portable devices converge. Although Wi-Fi, ZigBee, ANT and Bluetooth wireless technologies are poised to spur new consumer healthcare markets, there are some wireless challenges designers should consider as various wireless technologies are introduced into the home.
Connected personal health with the increasing incidence of chronic diseases, healthcare providers and employers are exploring new ways to motivate consumers to conveniently manage their conditions. With increasing consumer awareness of increasing financial responsibility for personal health, more and more consumers are investing in wireless health and fitness technologies for themselves and “aging independently” technologies for their parents… http://www.nxtbook.com/nxtbooks/hearst/projectmedical_vol3no1/index.php?startid=10
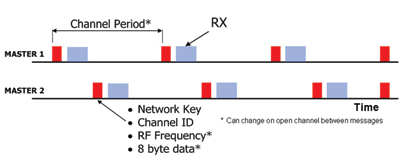
ANT is designed to allow coexistence in the time domain between multiple independent channels.
Choosing optical coatings for medical displays
BY JEFF BLAKE and RICHARD PAYNTON
Dontech, Doylestown, PA
www.dontech.com
Electronic displays are widely used in the healthcare industry for diverse applications such as patient monitoring, medical imaging diagnostics, archiving medical records, and EMT response. Because display image quality and reliability are critical to both the clinical and diagnostic integrity of patient care, design engineers are challenged to meet a wide range of optical, electrical and mechanical performance standards… http://www.nxtbook.com/nxtbooks/hearst/projectmedical_vol3no1/index.php?startid=16
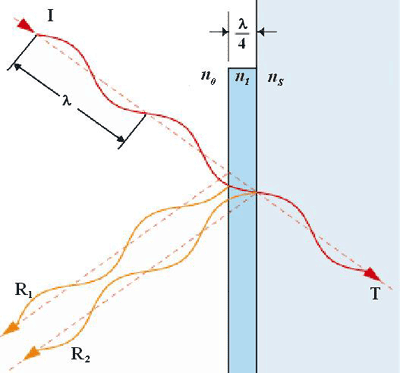
Graphical representation of how a simple, single-layer AR coating ( /4 optical thickness) with low, 1.38 refractive index creates destructive interference.
A Positive Pathway
One of the greatest challenges that medical and healthcare device manufacturers face when developing new products is obtaining FDA approval. Not only must they adhere to their strict regulations while designing, but they must also wait several months, sometimes years, for 510(k) approval in the United States – which basically means that today's patients will not benefit from today's cutting-edge technology.
But that may change in the near future.
Earlier this year, the U.S. FDA's Center for Devices and Radiological Health (CDRH) proposed the “Innovation Pathway,” a review program created to help bring breakthrough medical devices to the market faster. The program is just one part of the CDRH's “Medical Device Innovation Initiative,” a broader effort that seeks to encourage the development of cutting-edge technologies among medical device manufacturers and strengthen the nation's research infrastructure for developing breakthrough technologies and advancing quality regulatory science.
To date, the FDA has already accepted a submission from the Defense Advanced Research Projects Agency (DARPA) to review a brain-controlled, upper-extremity prosthesis designed to restore near-natural arm, hand, and finger function to patients suffering from spinal cord injury, stroke or amputation.
While the new Initiative sounds promising there are questions abound. Will this new program bring products to market faster? Or will it bring new bureaucratic hurdles? The FDA says that it could conduct premarket reviews of products in the Innovation Pathway within 150 days, nearly half the time it currently takes. Is this a realistic, sufficient amount of time? At this point in time, the FDA has yet to hear public feedback. Personally, I am curious to hear how this Pathway pans out over the next year or so. What do you think?
Christina D’Airo
For more on Project Medical, visit www.electronicproducts.com/projectmedical
Advertisement
Learn more about Digi-Key





