Relieving thermally induced stress with epoxies
A guide to selecting low-stress adhesives
BY DR. WALTER BRENNER
Master Bond
www.masterbond.com
Intelligent electronic devices have revolutionized our lives. Programmable appliances, personal communication devices, and navigation systems have changed the way we perform everyday tasks, while sophisticated computing, diagnostic, and control systems have enhanced our understanding of the world around us and within us. Rapid advances in microelectronics, optoelectronics, materials science, and software development have paved the way for innovative products ranging from feature-packed smartphones and e-readers to robotic surgical systems and unmanned aerial vehicles (UAVs).
High elongation adhesive compounds are frequently employed to bond dissimilar substrates exposed to thermal cycling.
The ever-increasing market demand for more powerful, versatile electronic devices has led to many physical changes in the underlying circuitry. Larger chips with higher I/O counts facilitate increased processing power and functionality. Fragile microelectromechanical systems (MEMS) augment traditional processing resources with sensing and control capabilities. Thinner silicon or gallium arsenide (GaAs) die (as low as several mils) make it possible to fit electronics into slimmer packages, while flexible circuitry enables assemblies to conform to a desired shape or flex during use.
Sophisticated assemblies carry increased risk of stress related failures
Many of today’s complex electronic assemblies are more sensitive to the effects of temperature excursions, shock, and vibration than their predecessors. Cracking, delamination, and other failures may occur, either immediately after assembly or later — after the device has been put into service. A key reason for many such failures stems from the bonding of dissimilar materials with widely different coefficients of thermal expansion (CTEs).
The coefficient of thermal expansion quantifies how much a material expands or contracts during temperature excursions, and is approximated as follows:
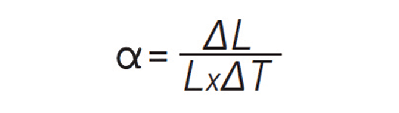
where α is the coefficient of linear thermal expansion, ΔL is the change in length of the material, L is the initial length of the material, and ΔT is the change in temperature. The CTE is a ratio of the change in length per degree temperature change to the initial length, and is usually reported as ppm/°C. The higher the CTE of a given material, the more it will expand or contract with temperature excursions. Since CTEs vary with temperature, they are usually given for a specific temperature range.
The equation above can be rearranged as follows:
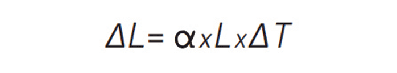
This equation shows that, for a given temperature excursion, the amount of expansion (or contraction) of a material is proportional to its CTE and to its initial length.
So components and substrates made of materials with different CTEs will expand and contract at different rates, and larger components will expand (or contract) more than smaller components.
This simple relationship illustrates the heart of the problem with bonding dissimilar materials in electronic assemblies. Temperature excursions cause the materials to expand and contract at very different rates. At each joint, as the bonded materials expand and contract, they push and pull on each other with different forces. These differential forces lead to stress build up — and that stress is relieved through cracking, warping, fracturing, and other failures.
Global CTE mismatches between electronic components and printed circuit boards (PCBs) can range anywhere from 2 to 14 ppm/°C for well-matched materials depending on the substrates. The table lists typical CTEs for a variety of materials commonly used in electronic assemblies. (See also the box, “The importance of the glass transition temperature“)
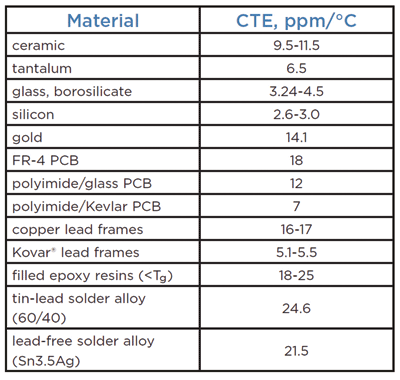
Typical coefficients of thermal expansion for various materials.
Stress also develops as a result of local CTE mismatches between the bonding material — whether a solder alloy or an adhesive — and the base material of the component or printed circuit board (PCB) to which it is attached. The CTE mismatch between Kovar lead frames and lead-free solder is approximately 16 ppm/°C. Although local CTE mismatches may be relatively large, their effects are small compared to those of global CTE mismatches, due to the fact that the stress-causing distortion is proportional to the length of the material, and bond lengths are typically small compared to component lengths.
The challenge for design engineers is to find ways to minimize or relieve stress in order to prevent damage to the assembly.
Special low-stress adhesive systems feature superior heat dissipation properties, exceptional temperature resistance and outstanding electrical insulation characteristics.
Minimizing thermal stress by design
The best way to relieve stress is to avoid it in the first place — by choosing materials with similar CTEs — but this is not always possible. Many components today are made with copper leadframes designed to minimize stress when joining the components to copper traces on a PCB.
However, the silicon die within these components has a much lower CTE than the copper leadframe, so thermal stress is still a concern. The more complex an assembly is, the more impractical it is to match the CTEs of all the materials that contact each other.
Additionally, in today’s electronic assemblies, several factors other than global CTE mismatches often contribute to thermally induced stress. The lower tensile strength of fragile materials, such as fiber optics and thinned silicon or GaAs die, can be overcome by stress caused by local CTE mismatches — resulting in damage to the component. Larger die incur increased stress proportional to their lengths, while flip-chips with hundreds of densely packed microbumps are subject to increased stress. Thermally induced stress in most modern electronic assemblies is simply unavoidable. For this reason, thermal stress management is an absolute necessity.
An alternative design strategy for managing thermal stress is to use stress-absorbing materials to bond and encapsulate components.
Low-modulus adhesives absorb and dissipate stress
Stress-absorbing materials are characterized by low moduli of elasticity and high elongation properties. The modulus of elasticity (also known as Young’s Modulus) is a measure of the stiffness of a material. Materials with low moduli are flexible, deforming more in response to a given stress than materials with high moduli. Harder materials tend to have higher moduli of elasticity than softer materials. When used to join dissimilar substrates, low-moduli stress absorbers take up the deflections of the adjoining materials, allowing the bonded entities to move more freely with little constraint. In essence, stress absorbers decouple the deflections of the adjoined materials — making them ideal for joining or encapsulating components subject to thermal stress.
Low-stress adhesives consist of epoxy, silicone, or urethane compounds selected for their low moduli and excellent elongation properties. Each type of compound offers a unique set of advantages. For example, silicone
formulations feature flexibility and high temperature resistance, while urethane systems offer flexibility, chemical and abrasion resistance, and fast cures. Low-stress adhesives can be engineered for a variety of design and performance requirements, such as temperature excursion limits (ΔT), adherence to specific substrate/component materials, and electrical conductivity requirements.
A common misconception among design engineers tasked with choosing a low-stress adhesive is that the CTE of the adhesive must be “somewhere in the middle,” between the CTEs of the adherends. While this would help alleviate thermally induced stress between dissimilar materials, it is far less effective than selecting an adhesive with a low modulus of elasticity. A flexible adhesive is quite capable of absorbing the deformation effects of global CTE mismatches, regardless of the adhesive’s CTE. Additionally, trying to match the CTE of the adhesive to those of the adherends may require selecting a different compound for each distinct combination of adherends in an assembly — unnecessarily complicating the design. ■
Box: The importance of the glass transition temperature
Selecting the optimum adhesive for a particular application requires an understanding of the properties of various compounds and substrates, how the application and environment affects the materials, and how bonded materials interact with each other during service operation. One of the most important parameters to consider is the glass transition temperature (Tg ).
For polymeric materials, such as epoxies and silicones, the CTE can change dramatically once the glass transition temperature of the material has been reached. The Tg is the temperature at which a significant physical change in the material takes place — from a rigid, glassy state (below the Tg ) to a soft amorphous state (above the Tg ). At and above theTg , the polymer molecules are less orderly and molecular motion increases. Consequently, temperature excursions above the Tg produce larger expansions than temperature excursions below the Tg . This is reflected in the CTE — which may be as much as five times higher above the Tg than below the Tg .
Adhesive manufacturers often report two CTE values: #1, the CTE value below the Tg , and #2, the CTE value above the Tg .Ideally, when selecting an epoxy adhesive for a particular application, its Tg should be higher than the upper temperature limit of the application for good bond strength and creep resistance. In practice, certain temperature excursions above the Tg are not problematic, depending upon the particular application. For example, when two metals are bonded by an adhesive subject to a 30-s wave solder process at 230°C (above its Tg ), the metals may act as heat sinks, drawing heat away from the adhesive and limiting the effects of the extreme temperature on the adhesive.
Silicone compounds have a very low Tg of 120°C, and maintain a low modulus of elasticity over a wide range of temperatures. At temperatures above the Tg , silicones offer tremendous flexibility and high temperature resistance — at the expense of other properties, such as bond strength and chemical resistance. Silicone compounds are often used to absorb stress for potting and encapsulation applications.
Cure conditions can also affect both the glass transition temperature and resultant stress. For instance, curing at higher temperatures for longer intervals can raise the Tg , resulting in a wider service temperature range. Overcuring, however, can make the compound brittle while degrading its modulus and flexural strength. And by allowing an adhesive to gel at significantly lower temperatures through step curing, stress within an adhesive bond can be greatly reduced. ■
Advertisement
Learn more about Master Bond





