For as long as lithium-ion batteries remain the staple form of battery, researchers will continue to try and squeeze in as much capacity as possible in an effort to prolong the technology’s lifespan. The latest breakthrough, which wound up quadrupling lithium-ion battery life, resulted from a lab accident.
The graphite anodes, which determine the lifespan of contemporary lithium-ion, tend to degrade over time from continuous use. Each time the battery charges and discharges, the anodes expand and contract as the lithium ions migrate, eventually resulting in a buildup of lithium compounds on the electrodes that break off and expose the surface of the electrode, making it liable to break down.
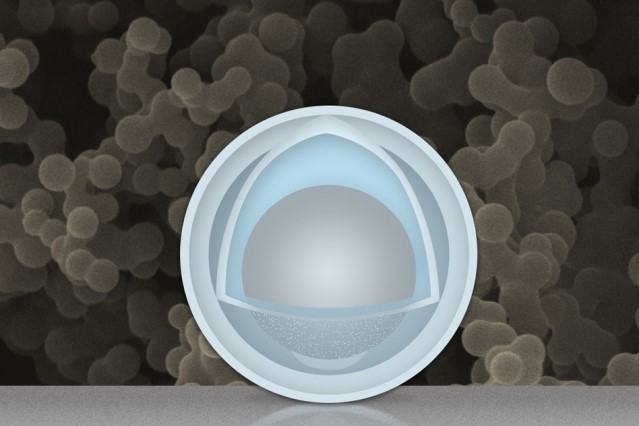
Aluminum is considered a super alternative to graphite, but until now, scientists have been unable to prevent the excessive expanding and contracting that occurs when creating anodes out of this material. Two scientists — Dr. Wang Changan of TsingHua University and Dr. Li Ju of MIT — successfully accomplished this and managed to stop the oxide coating that forms on the surface of aluminum particles that’ve been exposed to oxygen. By soaking up the aluminum nanoparticles in a mixture sulfuric acid and titanium oxysuplphate, the aluminum oxide slowly dissolves and becomes replaced with titanium oxide, a much hardier substance.
Constructing the new outer coating required key timing, but for whatever reason, the pair forgot to remove the batch from its bath for several hours, causing the acid to leak into the aluminum’s 50 nm nanoparticle shell, which then dissolved some of the aluminum inside creating a nanoparticle with a 4nm outer shell of titanium hydroxide and an inner 30 nm “yolk” of aluminum.
Surprisingly, the new compound did not expand or contract when used to create the anodes in a test battery, instead, yielded a battery that retained up to four-times the capacity of graphite-anode batteries even after testing it at 500 charge/discharge cycles. According to MIT, while graphite exhibits a storage capacity of 0.35 ampere-hours per gram (Ah/g), aluminum can theoretically provide a capacity of 2 Ah/g at a very low cost.
Dr. Li explains that the materials are inexpensive, allowing for scalable manufacturing that could provide a benefit for applications that require a high power and energy-density battery, adding “it’s probably the best anode material available.”
Advertisement
Learn more about Electronic Products Magazine





