The Department of Energy has set benchmarks for storage materials that would make hydrogen a practical fuel for light-duty vehicles. Layers of graphene separated by nanotube pillars of boron nitride may be a suitable material to store hydrogen fuel in cars, according to Rice University scientists. The Rice lab materials scientist Rouzbeh Shahsavari determined in a new computational study that pillared boron nitride and graphene could be a candidate.
Just as pillars in a building make space between floors for people, pillars in boron nitride graphene make space for hydrogen atoms. The challenge is to make them enter and stay in sufficient numbers and exit upon demand.
In their latest molecular dynamics simulations, the researchers found that either pillared graphene or pillared boron nitride graphene would offer abundant surface area (about 2,547 square meters per gram) with good recyclable properties under ambient conditions. Their models showed that adding oxygen or lithium to the materials would make them even better at binding hydrogen.
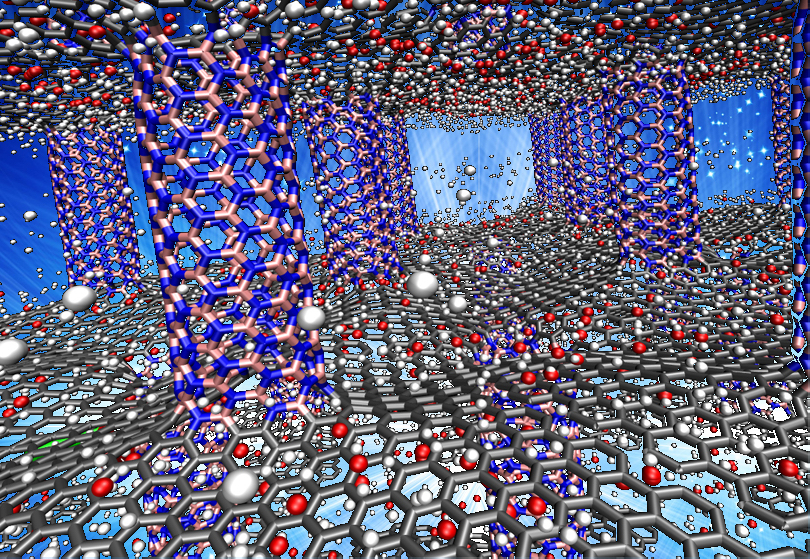
3D structures that combine boron nitride nanotubes and graphene may be suitable for hydrogen storage for cars.
They focused the simulations on four variants: pillared structures of boron nitride or pillared boron nitride graphene doped with either oxygen or lithium. At room temperature and in ambient pressure, oxygen-doped boron nitride graphene proved the best, holding 11.6% of its weight in hydrogen (its gravimetric capacity) and about 60 grams per liter (its volumetric capacity); it easily beat competing technologies like porous boron nitride, metal oxide frameworks, and carbon nanotubes.
At –321°F, the material held 14.77% of its weight in hydrogen. The Department of Energy’s current target for economic storage media is the ability to store more than 5.5% of its weight and 40 grams/liter in hydrogen under moderate conditions. The ultimate targets are 7.5% of its weight and 70 grams/liter.
Shahsavari said that hydrogen atoms adsorbed to the undoped pillared boron nitride graphene, thanks to weak van der Waals forces. When the material was doped with oxygen, the atoms bonded strongly with the hybrid and created a better surface for incoming hydrogen, which Shahsavari said would likely be delivered under pressure and would exit when pressure is released.
“What we’re looking for is the sweet spot,” Shahsavari said, describing the ideal conditions as a balance between the material’s surface area and weight, as well as the operating temperatures and pressures. “This is only practical through computational modeling because we can test a lot of variations very quickly. It would take experimentalists months to do what takes us only days.” He said that the structures should be robust enough to easily surpass the Department of Energy requirement that a hydrogen fuel tank be able to withstand 1,500 charge-discharge cycles. Find more information at http://tinyurl.com/nfdmzr5.
Advertisement





