Separating circulating cancer cells from blood cells for the purpose of diagnosing, prognosticating, and treating a patient, is about to become a lot easier thanks to the work of a team of researchers from Penn State University, Massachusetts Institute of Technology, Carnegie Mellon University, and the University of Notre Dame.
Presently, blood samples are put in a centrifuge for 10 minutes and spun at a rate of 3,000 revolutions per minute for the purpose of separating blood cells from cancer cells. One cancer-cell / blood-cell separation method includes the use tumor-specific antibodies that bind with the cancer cells and isolate them during the centrifuge spin; the problem here is they require that the appropriate antibodies be known prior to the procedure. Another approach is to scan the blood samples for size, deformability or electrical properties post spin cycle.
In short, it’s laborious work.
“Looking for circulating tumor cells in a blood sample is like looking for a needle in a haystack,” said Tony Jun Huang, professor of engineering science and mechanics at Penn State University. “Typically, the CTCs are about one in every one billion blood cells in the sample.”
The group’s aforementioned “sound” solution uses acoustic waves to separate cells in a non-invasive, much gentler way. It’s also much more cost-effective.
The researchers worked experimentally as well as with models to optimize the separation of cancer cells from blood. They used an acoustic-based microfluidic device that allowed a stream of blood to continuously pass through the device for separation. A bit more technically, two sound sources are placed opposite each other. They both emit the same wavelength of sound which creates a spot where they cancel one another out. Because they have pressure, these sound waves are able to be adjusted to push small objects, including cells and nanoparticles. The group adjusted the pressure of these sounds waves to a frequency capable of moving the cancer cells out of the blood stream; that is, as the blood passed through the device, the waves pushed the cancer cells out of the stream to the point where they cancelled each other out, or there was no lateral movement. The researchers specifically designed this “spot of cancellation” to be on a separate channel, so the cells could be easily collected for analysis.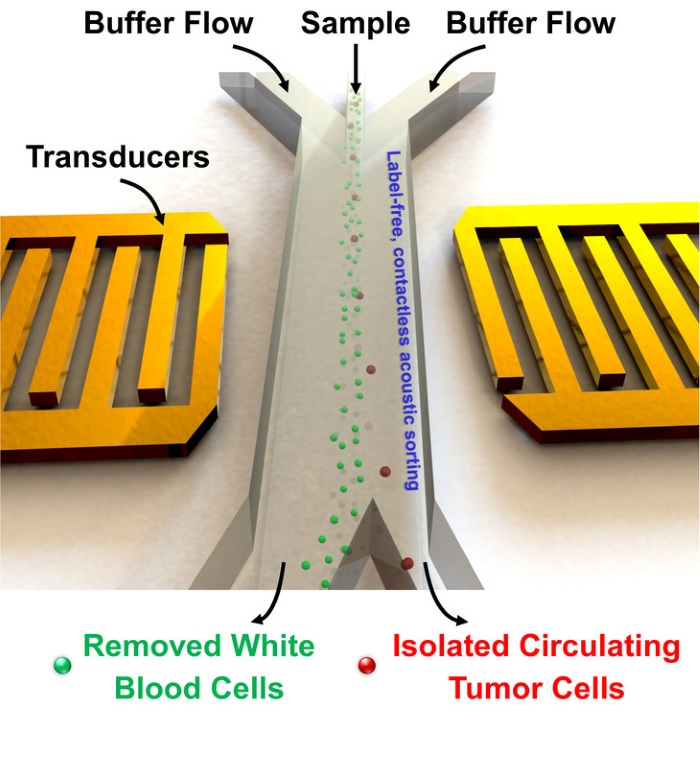
This technique uses very small amounts of energy — the power intensity and frequency used is similar to that which is used in ultrasonic imaging.
Also worth noting is that each cell is exposed to the acoustic wave for just a fraction of a second, thereby limiting any damaging effects to the blood sample as a whole.
For the researchers, this approach is much more efficient, as the cells do not require labeling or surface modification.
In terms of the results from the group’s experiments, two types of human cancer cells were used to test out their acoustic separation technique: HELA cells and MCF7 cells, which are similar in size.
Using their technique, they had a separation rate of more than 83%.
To further test their technique, the group separated other cancer cells without first optimizing the pressure of the acoustic waves to match the size / weight of the new cancer cells; again, they recorded a separation rate of more than 83%.
The team is now looking to take their device outside the lab.
“Because these devices are intended for use with human blood, they need to be disposable,” said Huang. “We are currently figuring out manufacturing and mass production possibilities.”
They also need to be rapidly and easily applicable if they’re ever going to see clinical use.
“In order to significantly increase the throughput for capturing those rare CTCs, device design has to be optimized for much higher flow rates and longer acoustic working length,” said Ming Dao, principal research scientist, materials science and engineering, Massachusetts Institute of Technology. “With an integrated experimental/modeling approach, the new generation of the device has improved cell sorting throughput more than 20 times higher than previously achieved and made it possible for us to work with patient samples.”
Down the road, the researchers foresee a day where their device could be used for initial diagnosis, determining treatment and prognosis, and to monitor how patients react to chemotherapy,
“This work, involving a highly cross-disciplinary group of medical doctors, engineers, computational biologists, and device experts, has led to the design and development of a label-free platform for identifying and separating CTCs while preserving the integrity of the cell,” said Subra Suresh, president, Carnegie Mellon University and part of the research team. “It promises to offer new avenues for basic research into the pathology and metastasis, and for clinical diagnosis of rare tumor cells.”
The results of the group’s study was published in the April 6th issue in the journal Proceedings of the National Academy of Sciences.
Advertisement
Learn more about Electronic Products Magazine





