Special nanotubes may up Li battery’s energy density
Combining sulfur-coated carbon cathodes, silicon nanowire anodes, and enhanced electrolyte could produce next-gen battery
Stanford associate professor of materials science and engineering Yi Cui and his group of research students have used nanotechnology to fabricate an improved battery cathode that promises to greatly increase the energy density of rechargeable lithium ion batteries. Cui suggests that putting sulfur-coated carbon cathodes and the silicon nanowire anodes his team previously developed together, and using an enhanced electrolyte, could produce the next-generation battery design for electric cars and grid-scale energy storage.
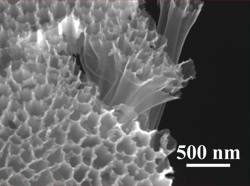
The hollow, carbon-nanofiber-encapsulated sulfur tubes seen in this scanning electron microscope photo make up the cathode at the heart of a new battery design by Stanford researchers. (Photo by Wesley Guangyuan Zheng.)
Cui noted that “Sulfur is one of the materials that can offer a 10-times higher charge storage capacity but with about half the voltage of the existing battery.” With the sulfur cathode as part of a complete battery, the higher charge capacity makes it possible to build a battery with four to five times the energy storage density of existing lithium ion battery technology.
Lithium-sulfur batteries have received attention previously because of their low cost and sulfur’s nontoxicity. But until now, lithium sulfur cathodes have rapidly failed from repeated charging and recharging, and so have not been practical. That’s because previous cathode designs coated the sulfur onto relatively open carbon structures, thereby exposing the sulfur to the battery’s electrolyte solution. This produced lithium polysulfides, which reduce the battery’s capacity by dissolving into the electrolyte. “This can be conflicting,” said Wesley Guangyuan Zheng, Cui’s graduate student, “because on the one side we don’t want a large surface area contacting the sulfur and the electrolyte, and on the other hand we want a large surface area for electrical and ionic conductivities.”
The new cathode design solves this problem by coating the inside of a hollow carbon nanofiber with sulfur, while leaving the outside uncoated. The length of a hollow nanofiber is about 300 times its diameter, and it is this nearly closed structure that prevents polysulfides from significantly leaking out into the electrolyte solution. Interestingly, the sulfur-nanofiber fabrication process uses a filter technology that is already commercially available and typically employed for water filtration.
Further, Cui’s graduate student Yuan Yang used an electrolyte additive that enhances the battery’s coulombic efficiency. “Without the additive you put 100 electrons into the battery and you get 85 out. With the additive, you get 99 out,” Cui said. For further information, phone Yi Cui at 650-723-4613 or e-mail
.
Richard Comerford
Advertisement
Learn more about Stanford University





