One problem with lithium-ion batteries is that they have the tendency to catch fire and blow up all kinds of gadgets, such as phones, toys, and even cars. To solve such an issue, a team of researchers from Stanford University created lithium-ion batteries with built-in fire extinguishers. They added a component called triphenyl phosphate — a compound commonly used as a flame retardant for electronics — to the plastic fibers of the part that keeps negative and positive electrodes separate.

The new method can stop batteries from burning up within 0.4 seconds.
Impressively, if the battery’s temperature reaches 150°C, the plastic fibers melt and release the chemical. Based on the researchers’ tests, the method can stop batteries from burning up within 0.4 seconds.
Previous attempts at building miniature fire extinguishers inside batteries hindered their performance. If a lithium-ion battery cell charges too quickly or a tiny manufacturing error slips through the net, it can result in a short circuit, which can lead to a fire. According to the project’s lead scientist, Yi Cui, his team’s batteries don’t have the same effect because the chemical won’t be released under normal temperatures.
“Using our ‘smart’ separators, battery electrochemical performance will not be affected by the flame retardant under normal conditions,” Cui said. “However, once there is a potential thermal runaway, the flame retardant will be activated and nip the fire or explosion in the bud.”
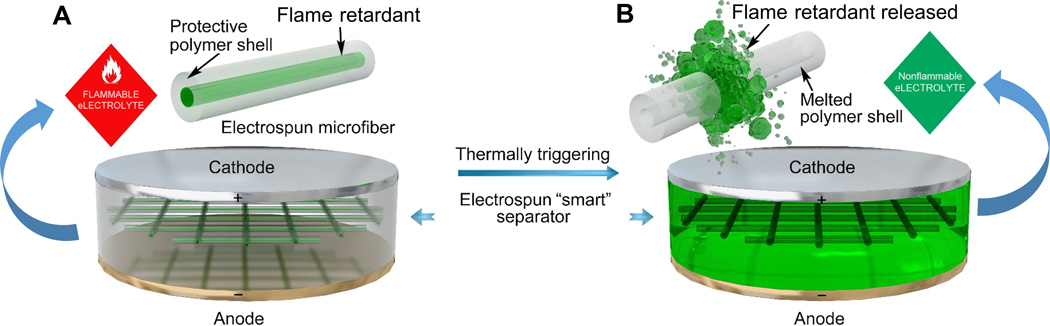
This figure shows the schematic of the smart electrospun separator with thermal-triggered flame-retardant properties for lithium-ion batteries.
As shown in the schematic, the free-standing separator is composed of microfibers with a core-shell structure, where the flame retardant serves as the core and the polymer is the shell. The encapsulation of the flame retardant inside the protective polymer shell has prevented direct exposure and dissolution of the flame retardant into the electrolyte, preventing their negative effects on the electrochemical performance of the battery. Upon thermal triggering, the polymer shell would melt and then the encapsulated flame retardant would be released into the electrolyte, effectively suppressing the ignition and burning of the electrolytes.
Continuing the search for better batteries
Unlike the many battery-powered devices that rely on battery technology, it’s been very slow to evolve. Right now, there’s great pressure to improve battery technology, and it’s one of the areas holding back mobile devices and a variety of other products.
Manufacturers have been balancing out consumer demand for longer-lived batteries and more powerful devices with better graphics and more detailed displays with the sophistication of battery technology. Still, it’s difficult to push up the capacity of batteries, and there will always be a risk that a battery in any device could fail.
The Stanford University research team's peer-reviewed paper has been published by the journal Science Advances.
Advertisement
Learn more about Electronic Products Magazine





