While the reason why Samsung Galaxy Note 7s have been catching fire has yet to be confirmed, the focus is on the device’s lithium-ion batteries. And those familiar with this technology have begun suggesting the battery might have a dendrite problem.
For those unfamiliar, dendrites are basically whiskers of lithium that grow inside batteries, and can cause the devices they’re powering to lost power more quickly, short out, or in some instances, catch fire.

Understanding dendrites and how to combat them is a relatively new field of study, and one that researchers at the University of Michigan have taken a keen interest in. They recently cut open a next-generation lithium battery (different from the lithium-ion power sources currently used in consumer products), and created a window through which to spy on the development and growth of the lithium strands. They’re hoping that over the course of their observation and study, they’ll be able to publish a better understanding on dendrites, from which combat solutions can be developed.
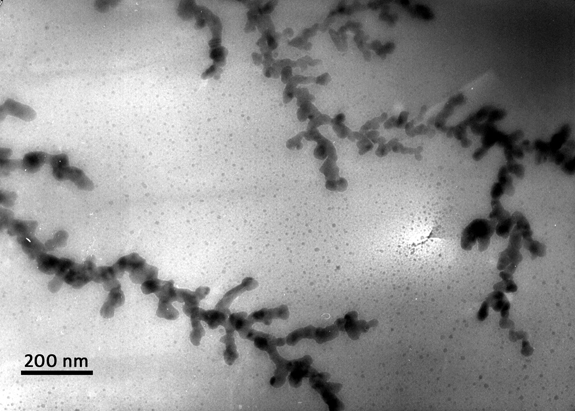
What’s already known about dendrites is based on studies of lithium metal batteries — a power source that got its name because they have all-metal electrodes. When they grow in the battery, it leads to a significant reduction in performance, while simultaneously raising safety concerns and cutting short the battery’s lifespan.
Some dendrites have even grown so fast and rigid that they’ve actually wound up piercing the membrane between the electrodes; this, in turn, shorted the lithium metal battery, and in some instances, led to spontaneous combustion.
The reason why it hasn’t been in the news is due to the fact that lithium metal batteries aren’t available on the market (yet). But these batteries and their lithium-ion counterparts are very similar to one another, which has led to many speculating that dendrites could be the cause behind the recent problems Samsung’s been having with its phones.
“As researchers try to cram more and more energy in the same amount of space, morphology problems like dendrites become major challenges. While we don't fully know why the Note 7s exploded, dendrites make bad things like that happen,” said Kevin Wood, a postdoctoral researcher in mechanical engineering who helped develop the battery window. “If we want high energy density batteries in the future and don't want them to explode, we need to solve the dendrite problem.”
The Michigan team is taking a different approach to understanding dendrites than other research groups have in the past. The latter bunch would focus on electromechanical measurements while the battery is in operation. After its run its course, an autopsy would be performed to understand what physical changes took place inside. With the Michigan approach, the team is hoping to understand not only how they grow, but why they’re growing.
The aforementioned “window” they created is outfitted with a high-definition video microscope and wired for easy monitoring of the dendrite growth and the voltage between the two electrodes (which changes during charge and discharge cycles). This allows the team to observe the electrode and record whether dendrites grew or shrunk, and the general state of degradation as it relates to the voltage measurements. From there, they were able to link voltage patterns to specific dendrite activity.
For what it’s worth, a battery with two lithium electrodes was used, a decision meant to avoid complicating the study by incorporating a different electrode, as it likely would’ve come with its own problems. From their observations, the team believes they now understand why dendrites grow in next-gen lithium metal batteries.
“Our window battery is a simple platform that can be used by researchers worldwide,” Dasgupta said. “It can be reproduced in any lab with an optical microscope, simple electrochemical equipment, a machine shop and a $100 budget.”
The team reports that dendrites grew as lithium accumulated on the surface of an electrode; it would shrink when the cycle reversed, as the lithium was being pulled away from the surface. They also saw pits from in the actual electrode when the lithium was removed, and saw how these pits wound up becoming nucleation sites for dendrites on the next cycle.
In terms of the physicality of the dendrites, the team described them as rather organic—much like plants growing and withering over the course of the battery’s cycle. Some actually broke off the electrode mid-cycle, and became “dead lithium” floating around the battery.
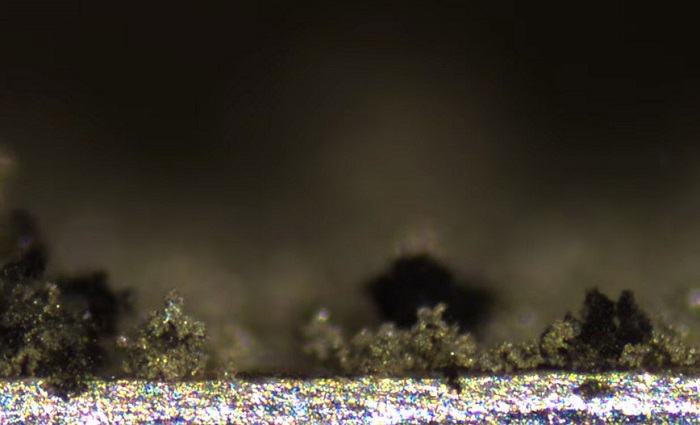
The group also reported that not all dendrites caused serious damage to the battery. For instance, smaller ones as well as those which evenly carpeted the surface of the electrode caused little-to-no damage, and battery performance remained stable.
“If you want to get to practical operating conditions, I don't think there's any way to truly prevent dendrite growth,” he said. “But by controlling dendrite growth you can enable batteries that have long lifetimes and better safety.”
To learn more, read the team’s published study, entitled “Dendrites and pits: untangling the complex behavior of lithium metal anodes through operando video microscopy”, which was published in ACS Central Science.
Via the University of Michigan University of Michigan
Advertisement
Learn more about Electronic Products Magazine





