Here’s help figuring out which battery type is right for your design
BY KERRY LANZA
Palladium Energy, Naperville, IL
www.palladiumenergy.com
Batteries are everywhere, and they play a large role in making our modern life possible. Every day, new battery chemistries and designs are announced that offer very high energy densities, deliver 1,000 charge/discharge cycles and are paper-thin. These attributes are indeed achievable, but not at the same time and not using the same battery.
A battery may be designed for small size and long runtime, but has a limited cycle life. Another may be built for durability and is big and bulky. A third may have high energy density and long durability, but is made for a special application and is too expensive for the average consumer.
Battery manufacturers are aware of customer needs and offer battery packs that best suit the application. The mobile phone industry is a good example of this change. Here, small size and high energy density are favored over longevity. Although energy density is the main concern, other important attributes include service life, load characteristics, maintenance requirements, self-discharge, cost, and safety. Nickel-cadmium was the first rechargeable battery in small format and forms a standard against which other chemistries are commonly compared. Today however, the most popular designs are lithium-based systems. See Table 1 for comparison data of six different battery types.
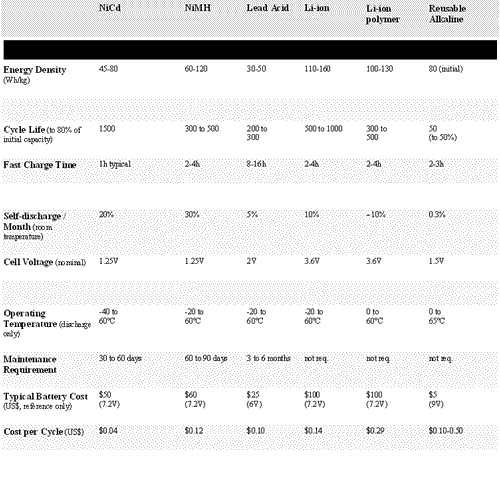
Table 1. Battery types and typical specifications
Nickel-cadmium battery
Nickel-cadmium (NiCd) prefers fast charge to slow charge and pulse charge to DC charge. It is a strong worker and hard work poses little problem. In fact, nickel-cadmium is the only battery type that performs well under rigorous working conditions – power tools for example. Nickel-cadmium does not like to be sitting in chargers for extended times and being used for short periods. A periodic full discharge is so important that, if omitted, large crystals will form on the cell plates (also referred to as memory) and the nickel-cadmium will gradually lose its performance.
NiCd remains a popular choice for two-way radios, emergency medical equipment and power tools. There is shift towards batteries with higher energy densities and less toxic metals but alternative chemistries cannot always match the superior durability and low cost of nickel-cadmium.
Here is a summary of the advantages and limitations of NiCd batteries.
Advantages
• Simple storage and transportation . Most airlines accept nickel-cadmium without special conditions.
• Forgiving if abused . NiCd is rugged.
• Good value . NiCd batteries are economically priced, and have a fast and simple charge, even after prolonged storage.
• Long life . Nickel-cadmium has a high number of charge/discharge cycles and five-year storage is possible.
• Good load performance . Nickel-cadmium allows recharging at low temperatures.
• Wide variety . NiCd is available in a wide range of sizes and performance options.
Limitations
• Environmentally unfriendly . NiCd contains toxic metals.
• Relatively high self-discharge . Nickel cadmium needs recharging after storage.
• Low energy density . NiCd batteries are larger and heavier than alternatives.
• Memory effect . NiCd must periodically be exercised.
Nickel-metal-hydride battery
The success of nickel-metal hydride (NiMH) has been driven by high energy density and the use of environmentally friendly metals. The modern NiMH offers up to 40% higher energy density compared to the standard nickel-cadmium. There is potential for yet higher capacities, but not without some negative side effects.
NiMH is less durable than NiCd. Cycling under heavy load and storage at high temperature reduces the service life. NiMH experiences high self-discharge, which is higher than that of NiCd. Most shortcomings are similar to the nickel-based technology and are shared with NiCd. It is widely accepted that NiMH is an interim step to lithium-based battery technology.
Advantages
• Simple storage and transportation . Transport is not subject to regulatory control.
• Environmentally friendly . NiMH contains only mild toxins.
• High capacity . NiMH has a 30% to40% higher capacity than NiCd.
• Less prone to memory. NiMH does not always need to be totally drained before recharging.
Limitations
• More complex charge algorithm needed .
• High self-discharge . NiMH discharge rate is typically 50% higher than NiCd.
• Heat sensitive . Performance of NiMH degrades if stored at high temperatures.
• Limited service life . The performance starts to deteriorate after 200 to 300 cycles if repeatedly deeply cycled.
• Short shelf life . NiMH has a relatively short storage of three years.
• Limited discharge current . Although NiMH is capable of delivering high discharge currents, heavy load reduces the battery’s cycle life.
• High maintenance . Nickel-metal hydride requires regular full discharge to prevent crystalline formation.
Lithium-ion battery
The energy density of lithium-ion is typically twice that of the standard NiCd. The high cell voltage of 3.6 – 4.2 volts allows battery pack designs with only one cell. Most of today’s mobile phones run on a single cell. A nickel-based pack would require three 1.2-V cells connected in series.
Lithium-ion is a low maintenance battery, an advantage that most other chemistries cannot claim. There is no memory and no scheduled cycling is required to prolong the battery’s life. In addition, the self-discharge is less than half compared to nickel-cadmium, making lithium-ion well suited for modern fuel gauge applications. Lithium-ion cells also cause little harm when disposed.
Despite its overall advantages, lithium-ion has its drawbacks. It is delicate and requires a protection circuit to maintain safe operation. The built-in protection circuit limits the peak voltage of each cell during charge and prevents the cell voltage from dropping too low on discharge. Also, the cell temperature is monitored to prevent temperature extremes. The maximum charge and discharge current on most packs is limited to between 1C and 2C. With these precautions in place, the possibility of metallic lithium plating occurring due to overcharge is virtually eliminated.
Manufacturers recommend storage temperatures of 15°C (59°F), because storage in a cool place slows the aging process of lithium-ion (and other chemistries). Manufacturers of lithium cells recommend a 40% charge level for storage.
Advantages
• Low maintenance . No periodic discharge is needed; there is no memory.
• Variety of use . Specialty cells can provide very high current to applications such as power tools.
• High energy density . There is potential for even higher capacities.
• Relatively low self-discharge. Self-discharge is less than half that of nickel-based batteries.
Limitations
• Safety issues . Lithium-ion requires protection circuit to maintain voltage and current within safe limits.
• Subject to aging. Aging occurs even when the battery is not in use, but storage in a cool place at 40% charge reduces the effect.
• Transportation limitations . Shipment of larger quantities may be subject to regulatory control.
• Expensive to manufacture . Lithium-ion costs are about 40% higher than NiCd.
Lithium polymer battery
The lithium-polymer differentiates itself from conventional battery systems in the type of electrolyte used. This electrolyte resembles a plastic-like film that does not conduct electricity but allows ions to exchange. The polymer electrolyte replaces the traditional porous separator, which is soaked with electrolyte. The commercial lithium-ion polymer cells are very similar in chemistry and materials to their liquid electrolyte counter parts.
Lithium-polymer has not caught on as quickly as some analysts had expected. Low manufacturing costs, for example, have not been totally realized. Because lithium-polymer cells are easier to deform and damage than standard lithium-ion cells, more consideration has to be given in the device design and on how the user will access, use, and replace the battery. Nevertheless, lithium-polymer has found its market niche in thin geometries.
Advantages
• Lightweight . Lithium polymer, with its high charge density, weighs less than the equivalent energy density of nickel-based and lithium-ion batteries.
• Improved safety . Lithium-polymer is more resistant to overcharge, so there is less chance for electrolyte leakage.
• Very low profile . Batteries resembling the profile of a credit card are feasible.
• Flexible form factor . Manufacturers are not bound by standard cell formats.
Limitations
• Different safety concerns . Many device designers who use lithium-polymer have found it safer to make the battery pack nonreplaceable in order to prevent potential consumer damage to the more fragile battery pack.
• Lower energy density and decreased cycle count compared to lithium-ion. Lithium polymer cells are smaller for an equivalent output and can be charged-discharged more frequently.
• Expensive to manufacture . Lithium polymer technology is complex and manufacturing costs are higher than other technologies.
• No standard sizes . Most cells are produced for high volume consumer markets.
• Expensive . There is a higher cost-to-energy ratio than with lithium-ion. ■
Related Products: Batteries
Advertisement
Learn more about Palladium Energy





