When to choose lithium-iron phosphate batteries
LiFePO4 suit rugged applications and provide superior safety over Li-ion
BY BRIDGET DEVENEY
Saft, Cockeysville, MD
http://www.saftbatteries.com
There are many factors to consider when designing the battery into an electronic device, especially when that piece of equipment could impact the operator’s life. Designers have myriad choices in terms of battery chemistry, size, power, cost and safety. The goal is to prioritize these options based on the needs of the application. It is critical to work with a qualified manufacturer from the beginning of the design process to ensure the battery will meet all of your performance needs and come in at an acceptable cost.
Lithium-ion (Li-ion) batteries have become a popular choice for many designers because they offer qualities making them ideal for jobs demanding high power and energy without additional weight. Small Li-ion cells can be found in various consumer electronics and larger cells are commonly used in commercial applications.
Compared to other battery technologies, such as nickel, lead and silver, Li-ion has several advantages. Because lithium is the lightest metal and has a high electrochemical potential, lithium-ion provides more energy per volume and operates at higher voltages to maximize the power-to-weight ratio of a battery. Li-ion batteries can also be recharged repeatedly, which reduces their life cycle costs compared to some other chemistries. Because of these advantages, Li-ion battery technology is increasingly being used in a number of military applications. The development of Li-ion was a significant step forward in safety from the previously used lithium metal rechargeable technology; however, Li-ion can still be reactive. This has made the adoption of Li-ion technology less likely for some military applications, particularly when exposure to extremely harsh abuse is possible. In order to offer an alternative to Li-ion that maintains most of the same benefits of the technology, some battery manufacturers have been considering lithium-iron phosphate technology.
What is lithium-iron phosphate technology?
Traditional Li-ion batteries are made with transition metal oxide cathode materials such as lithium-cobalt oxide or lithium-nickel-cobalt aluminum oxide. Lithium-iron phosphate (LiFePO4 ) batteries are rechargeable Li-ion batteries that use LiFePO4 as the cathode material.
This low cost, naturally-occurring mineral offers excellent thermal stability, very fast charge times and long cycle life, but with some loss of energy content because it operates at a slightly lower voltage than standard Li-ion chemistry. The use of iron phosphate material in Li-ion batteries was first discovered and patented in 1997 by Dr. Goodenough from the University of Texas.

Lithium-iron phosphate is being considered for a variety of applications including grid stabilization, power tools, hybrid electric vehicles, lasers, naval operations, planes and helicopters. There are advantages and disadvantages to using iron phosphate instead of standard Li-ion (with metal oxide cathode material). How do you make the best choice for the application? It all comes down to how you rank performance, safety and cost.
The primary advantage of iron phosphate over metal oxide based Li-ion is increased abuse tolerance, because the product is less prone to reactivity under such conditions. Additional benefits of iron phosphate include a longer cycle life, faster recharge, and low cost of raw materials.
Like all tradeoffs, the iron phosphate chemistry comes with its disadvantages when compared to traditional metal oxide based Li-ion, such as less energy for a given volume/weight, more sensitivity to storage at elevated temperatures (although better than lead acid, Ni-Cd or Ni-MH), and poorer performance at low temperature.
Interestingly, iron phosphate’s flat voltage profile becomes both an advantage and a disadvantage for designers. On the positive side, the cell provides constant power delivery within a tight voltage window over 80% of the state-of-charge (SOC) and storing the battery fully charged has minimal impact on its life. For an application with a narrow voltage window, this utilized energy can be maximized for the most efficient use. However, because battery voltage is not an indicator of SOC or remaining energy as in a metal oxide based Li-ion, having a reliable gas gauge for a LiFePO4 battery can be more complicated.
Although iron phosphate technology has some obvious drawbacks, scientists have conducted research and development to overcome them and capitalize on its advantages. As a result, some battery manufacturers have developed advanced iron phosphate technology that improves the power to energy tradeoff found in standard iron phosphate. For example, Super-Phosphate is Saft’s proprietary patent pending iron phosphate-based chemistry with all the safety benefits, higher power and higher energy. The battery, module and mechanical cell design for cells containing the iron phosphate chemistry are identical to cells containing Saft’s standard Li-ion chemistry using lithium nickel cobalt aluminum oxide, so for most applications, Super-Phosphate is interchangeable with traditional Li-ion or lithium-iron phosphate in system hardware.
Compared with standard lithium-iron phosphate cells, Super-Phosphate technology features proven safety, longer cycle life, better calendar life and a wide operating temperature range, including superior lower temperature performance (see Fig. 1 ). Additionally, the cells have a high resistance to abuse and can safely accept a regenerative charge from float conditions.
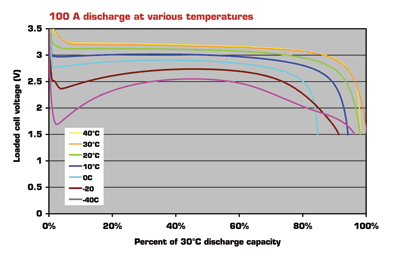
Fig. 1. 100-A discharge for SuperPhosphate at various temperatures.
Unlike most lithium-iron phosphate batteries, Super-Phosphate chemistry is capable of accepting significant charge current while floating on a bus at slightly less than 100 percent SOC. Most iron phosphate batteries are unable to float on a bus at anything less than a fully charged state.
From that condition, even a brief high current charge pulse can lead to an overcharge condition. This is important for applications that require regenerative charge current, such as motor control with dynamic braking, electrical actuator control, or dc/dc converters that require an energy sink if load current is abruptly stopped. The ability to recharge from float conditions improves the performance of electronic systems, overcharge tolerance, and allows for superior cell balancing for easier battery maintenance.
Iron phosphate apps
Because of the safety benefits of iron phosphate, combined with its high power output and high energy density (compared to other battery technologies like lead-acid, NiMH, and NiCd), many battery manufacturers are using it for power tools and grid stabilization. These rugged iron phosphate cells are ideal for large defense applications, specifically those with stringent safety requirements such as underwater operations and aviation. In such applications, extreme abuse from uncontrolled environmental conditions may occur, demanding additional safety levels.
Iron phosphate, like any battery chemistry, has positive and negative points and advanced versions, like Saft’s Super-Phosphate, maintain safety, while improving power, energy and performance at low temperatures. When it comes to safety considerations, it’s important to remember cell level safety is only part of a comprehensive system design. The cells should withstand common field abuses, such as overcharge, mechanical shock and vibration, as well as external short-circuit, crushing, penetration and more. Because each country does not always define safety standards, it is best to meet the highest standard and ensure compatibility with customer requirements and expectations. ■
Advertisement
Learn more about Saft Batteries





