Designing and developing a medical device is no small feat, especially when the goal of the device is to help eliminate medication errors and save lives. Yet, in the long process of bringing its new infusion system to market, Ivenix, Inc., located in Amesbury, MA, found it could dramatically save time, lower costs, and reduce headaches by selecting a PCB assembly partner focused on helping customers make their ideas a reality.
Ivenix’s first offering—a large volume infusion system—is intended to deliver a dramatically higher level of safety and improved workflow optimization over the infusion pumps being used today.
The Challenge
When working with infusion pumps, the causes of potential errors—whether from human error, difficulties with interfaces, or hardware failures—can be extensive. Unfortunately, these types of problems account for a large number of incidents resulting in patient harm and even death. According to a New York Times article, entitled F.D.A. Steps Up Oversight of Infusion Pumps, the F.D.A. Center for Devices and Radiological Health reported 710 deaths total and more than 10,000 infusion-pump complaints each year between 2005 and 2009. Infusion pump manufacturers also issued 79 recalls during that same timeframe. There have been 70 additional Class I and Class II recalls since, placing infusion pump recalls among the highest for any medical device.
Typically, infusion pumps are gravity dependent, peristaltic, and open-loop in nature. Users must enter data manually, and there are typically few safeguards or checks in place to ensure medication is administered accurately to a patient. Ivenix intends to change all that. The Ivenix infusion system utilizes a pneumatic pump technology that will deliver medications under all clinical conditions, regardless of the height of the bag or proximity of the pump to the patient.
Getting such a pump to market, however, is subject to three main challenges. First, the size of the device must be compact and portable. Developing electronic devices in parallel to the mechanical design so that the electronics can fit into the “box,” however, can be problematic. Second, the device must be able to work consistently at a high level of reliability. And finally, the fact that the device is enhanced with the latest and greatest technology does not mean that the cost can be out of line with traditional solutions, especially as healthcare institutions are continually faced with pressures to cut costs wherever possible while improving patient care.
Adding Technology
The Ivenix closed-loop, self-aware system, coupled with a smartphone-like user interface, features multiple redundant processors, wireless connectivity, and IT capabilities that the company believes will improve patient safety, improve the clinician’s workflow, and lower the total cost of ownership.
Launching the next-generation infusion system program back in 2013, the company set out to create a prototype that would work—no matter the size or cost. Ivenix recently wrapped up the design phase of the project, and the device, though not yet commercially available, is now production-ready. In preparation for F.D.A. submission next year, Ivenix is performing the vast number of tests necessary to bring a medical device to market.
In order to reach the milestones necessary to bring a new medical device to market, Ivenix had to find a way to consistently produce numerous revisions of circuit boards that would enable system evaluation throughout the design process.
The Solution
When John Yannone, Principal Electrical Engineer at Ivenix, joined the company, he began a search for a contract manufacturer that could deliver fast turnaround at a price point they could afford, without compromising on quality. Initially, the company attempted to modify a predecessor platform that gave way to the system behind the device that they will bring to market.
“One thing I was looking for was a way to get boards in hand more quickly, so I called a bunch of companies and began asking a long list of questions. One company stood out. For every question I asked, Advanced Assembly came back with positive answers. This went on for days. Since I was happy with all of their answers, I decided to give them a shot,” said Yannone.
“We selected their first article option in which Advanced Assembly would overnight the first three assemblies to us after only three days assembly time. That was obviously a huge time saver for us, which made it very attractive,” he added.
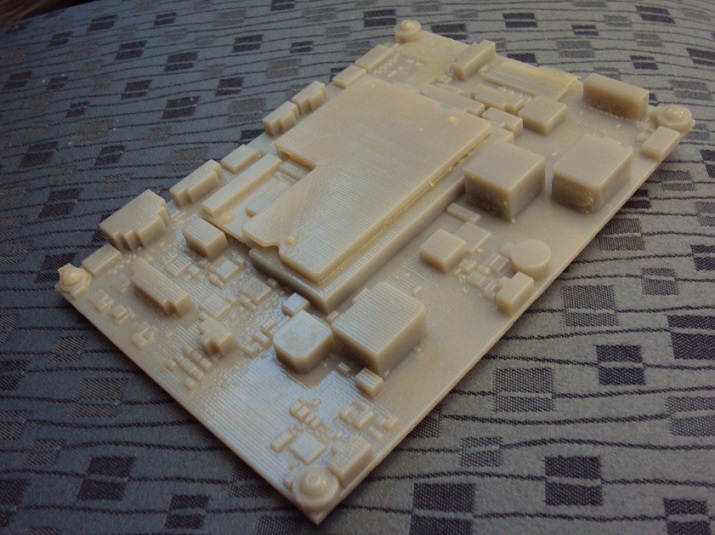
Figure 1: To speed things along, the company uses a 3-D printer regularly, printing parts to ensure components fit together properly. (Source: Ivenix)
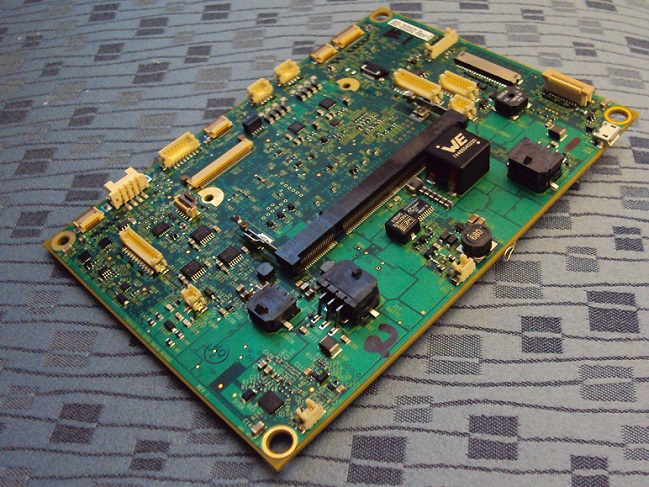
Figure 2: The actual board manufactured by Advanced Assembly. (Source: Ivenix)
After the first few prototype cycles, Ivenix was hooked. Ivenix would deliver the design files to Advanced Assembly with the knowledge and peace of mind that they would receive a board from Advanced Assembly in less than a week. This turnaround time shaved weeks off of the delivery times Yannone had grown accustomed to, having used other contract manufacturers in the past.
“Other companies did not deliver as quickly, or, they didn’t have the inspection capabilities we needed. Advanced Assembly, for example, offered automated optical inspection. While these added processes do take a little more time and money, these safeguards can really go a long way to reduce the number of defects, and—as a result—time involved in bringing up a new design.”
When asked about the value provided by Advanced Assembly for a startup enterprise such as his own, Yannone didn’t hesitate.
“I’ve always used quick-turn board shops for the raw boards. My experience is that it takes approximately two weeks to receive assembled boards after placing my order with a typical contract manufacturer. With Advanced Assembly, I really do get fast turnaround. In addition, customer support has been outstanding. Just the quoting alone, for example: I’ve sent a design over in the morning and have received a quote by the end of the day. That’s something that’s often overlooked elsewhere, and I’ve had to wait up to a week with other vendors just to get that quote.”
Yannone also appreciates Advanced Assembly’s operational efficiencies, citing experiences with other companies that left them with $20,000 in extra parts at the end of a project as a result of how the vendor purchased them.
“With Advanced Assembly there may be some leftover parts, but it’s not tens of thousands of dollars’ worth,” he added.
Since 2013, Ivenix has taken delivery of 482 assembled boards from Advanced Assembly that vary in complexity from a large board packed with electronics to just a small circuit board with three components. They are now also ordering and receiving flex circuits.
Ivenix has moved from “just making something work” to perfecting a variety of subsystems. Their infusion pump, for example, includes a power subsystem, a fluid-control subsystem, a safety subsystem, a user-interface subsystem, and communications systems. Key components are associated with each subsystem, making the entire design extremely sophisticated.
Yannone sums up his experience saying, “I have a high level of confidence that Advanced Assembly can assemble anything I throw at them and do it fast. They have the capabilities to assemble very complex boards, and they provide an amazingly high level of information and communication.”
For startups pushing the limits of development velocity, rapid prototyping is critical. Advanced Assembly has been a key vendor supporting the Ivenix team in their mission to bring a truly lifesaving technology to market.
Ivenix’s infusion pump is not yet commercially available, nor has it been reviewed or approved by the FDA or any similar foreign regulatory authority.
By: Carolyn Mathas
Advertisement
Learn more about Advanced Assembly





