By Gary Elinoff, contributing writer
Solar and wind energy are abundantly available worldwide at no cost, but the big problem is the difficulty in storing it. Mechanical devices such as flywheels are inefficient. Lithium-ion batteries are difficult to produce and bulky, and, as is highlighted all too often in the news, they are unstable, prone to catching fire and to explosion. And supercapacitors , the new star on the scene, can’t as yet store enough energy for their size and weight.
Enter hydrogen
One possible way to store electrical energy is to convert it into hydrogen gas, which can later be used as fuel for a fuel cell. The great thing about hydrogen-powered fuel cells is that their only “exhaust” is water, which makes them an almost environmentally perfect power source for electronics or perhaps even for electric vehicles.
Scientists at UCLA have developed a device whose front end is a solar cell that can capture the energy from the sun’s rays and either store it directly in a supercapacitor as electricity or convert it into the element hydrogen — a sort of technological two-for-one. As illustrated on the left side of the illustration below, the solar cell’s electrical output can be used to charge the internal supercapacitor. On the right side, the power garnered from the sun by the solar cell is used to split water into its components, generating hydrogen gas.
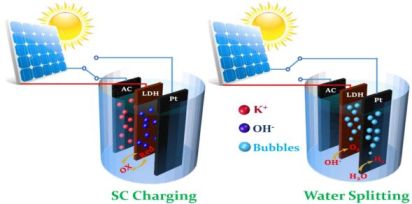
The device can function as a supercapacitor or as a generator of H2 gas. Image source: Energy Storage Materials.
Hydrogen is often misunderstood as an environmentally friendly fuel. If an electric vehicle were to be powered by hydrogen, it is true that the “exhaust” would consist only of harmless water. But as noted by UCLA postdoc Maher El-Kady, who worked on the UCLA project, most hydrogen used worldwide is produced using natural gas in a process that releases a great deal of carbon dioxide into the atmosphere. But when the hydrogen is produced from electricity supplied via solar cell, as it is here, there is no pollution generated in producing the hydrogen, just as there is not pollution caused by its use as a fuel.
As pointed out by Professor Richard Kaner, the project leader at UCLA, combining a solar cell, a supercapacitor, and a hydrogen-generating water splitter into one package is akin to “the first time a phone, web browser, and camera were combined on a smartphone.” According to Kaner , people need fuel to run their vehicles and electricity to run their devices, and with the new invention, you can make both electricity and fuel with a single device.
As illustrated below, the device produced at UCLA is quite small, but because it’s inherently inexpensive to produce, it can be readily scaled up as needed.

Image source: UCLA.
“If you could convert electricity to hydrogen, you could store it indefinitely,” said Professor Kaner. No matter how long it’s stored, hydrogen stays hydrogen, just as oil is always oil. This is unlike a power stored in a battery, which begins dissipating immediately.
Made possible by nanotechnology
The electrodes in this new device are formed at nanoscale, as small as one atom thick. This makes it possible to pack a vast amount of electrode area in a tiny volumetric space. For that reason, a relatively huge amount of electricity can be stored in the supercapacitor subsection and a high quantity of hydrogen gas can be generated in the water-splitting unit. The major elemental components of the device are nickel, iron, and cobalt, which are cheap and easy to deal with, unlike troublesome lithium.
Advertisement
Learn more about Electronic Products Magazine








